Norethisterone: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
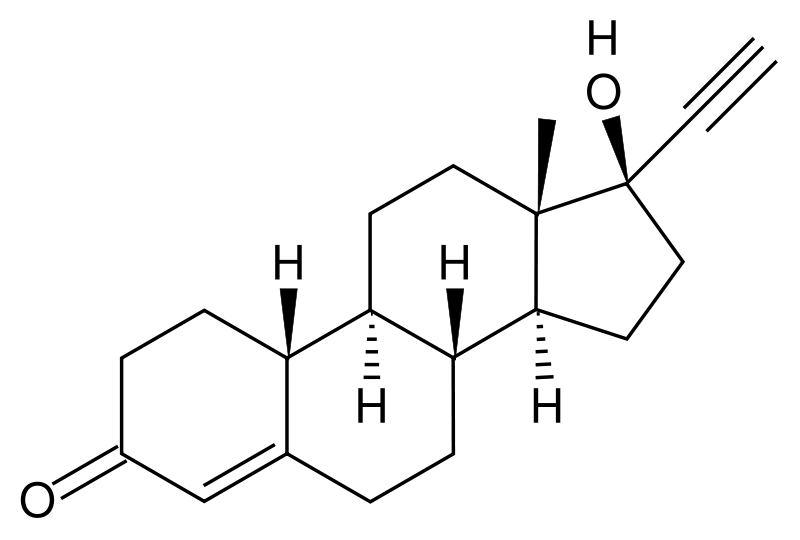
{{drugbox | IUPAC_name = (17β)-17-ethynyl-17-hydroxyestr-4-en-3-one |synonyms = <small>(8''R'',9''S'',10''R'',13''S'',14''S'',17''S'')-17-ethynyl-17-hydroxy-13-methyl-1, 2,6,7,8,9,10,11,12,14,15, 16-dodecahydrocyclopenta[''a'']phenanthren-3-one</small> | image = Norethisterone.svg | CAS_number = 68-22-4 | ATC_prefix = G03 | ATC_suffix = AA05 | ATC_supplemental = | PubChem = 6230 | DrugBank = APRD00679 | C = 20 | H = 26 | O = 2 | molecular_weight = 298.419 g/mol | bioavailability =64% | protein_bound = >95% | metabolism = | elimination_half-life = 7 hours | excretion = | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | pregnancy_US = <!-- A / B / C / D / X --> | pregnancy_category = | legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | legal_UK = <!-- GSL / P / POM / CD --> | legal_US = <!-- OTC / Rx-only --> | legal_status = | routes_of_administration = }} '''Norethisterone''' (or '''norethindrone''') (or 19-nor-17α-ethynyltestosterone) is a molecule used in some [[combined oral contraceptive pill]]s and in some [[progestogen only pill]]s. It is a [[progestogen]] and can be used to treat premenstrual syndrome, painful periods, abnormal heavy bleeding, irregular periods, menopausal syndrome (in combination with oestrogen), or to postpone a period. Norethindrone was the first orally highly active [[progestin]] to be synthesized. It was synthesized for the first time by chemists Luis E. Miramontes, Carl Djerassi, and George Rosenkranz at Syntex in Mexico City in 1951.<ref>{{cite journal |author=Djerassi C, Miramontes L, Rosenkranz G, Sondheimer F |year=1954 |title=Steroids. LIV. Synthesis of 19-Nor-17α-ethynyltestosterone and 19-Nor-17α-methyltestosterone |journal=J Am Chem Soc |volume=76 |issue=16 |pages=4089–4091 |url=http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/1954/76/i16/f-pdf/f_ja01645a010.pdf |doi=10.1021/ja01645a009 |format=abstract page}}</ref> It was the progestin used in one of the first two [[combined oral contraceptive pill| oral contraceptive]]s. It is often used as the related ester, [[norethisterone acetate]]. | {{drugbox | IUPAC_name = (17β)-17-ethynyl-17-hydroxyestr-4-en-3-one |synonyms = <small>(8''R'',9''S'',10''R'',13''S'',14''S'',17''S'')-17-ethynyl-17-hydroxy-13-methyl-1, 2,6,7,8,9,10,11,12,14,15, 16-dodecahydrocyclopenta[''a'']phenanthren-3-one</small> | image = Norethisterone.svg | CAS_number = 68-22-4 | ATC_prefix = G03 | ATC_suffix = AA05 | ATC_supplemental = | PubChem = 6230 | DrugBank = APRD00679 | C = 20 | H = 26 | O = 2 | molecular_weight = 298.419 g/mol | bioavailability =64% | protein_bound = >95% | metabolism = | elimination_half-life = 7 hours | excretion = | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | pregnancy_US = <!-- A / B / C / D / X --> | pregnancy_category = | legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | legal_UK = <!-- GSL / P / POM / CD --> | legal_US = <!-- OTC / Rx-only --> | legal_status = | routes_of_administration = }} '''Norethisterone''' (or '''norethindrone''') (or 19-nor-17α-ethynyltestosterone) is a molecule used in some [[combined oral contraceptive pill]]s and in some [[progestogen only pill]]s. It is a [[progestogen]] and can be used to treat premenstrual syndrome, painful periods, abnormal heavy bleeding, irregular periods, menopausal syndrome (in combination with oestrogen), or to postpone a period. Norethindrone was the first orally highly active [[progestin]] to be synthesized. It was synthesized for the first time by chemists Luis E. Miramontes, Carl Djerassi, and George Rosenkranz at Syntex in Mexico City in 1951.<ref>{{cite journal |author=Djerassi C, Miramontes L, Rosenkranz G, Sondheimer F |year=1954 |title=Steroids. LIV. Synthesis of 19-Nor-17α-ethynyltestosterone and 19-Nor-17α-methyltestosterone |journal=J Am Chem Soc |volume=76 |issue=16 |pages=4089–4091 |url=http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/1954/76/i16/f-pdf/f_ja01645a010.pdf |doi=10.1021/ja01645a009 |format=abstract page}}</ref> It was the progestin used in one of the first two [[combined oral contraceptive pill| oral contraceptive]]s. It is often used as the related ester, [[norethisterone acetate]]. | ||
== References == | |||
{{reflist}} | |||
{{Sex hormones}} | |||
[[Category:Syntex]] | |||
[[Category:Progestagens]] | |||
[[de:Norethisteron]] | |||
[[es:19-noretisterona]] | |||
[[pt:Noretindrona]] | |||
Revision as of 00:27, 23 April 2009
 | |
| Clinical data | |
|---|---|
| Synonyms | (8R,9S,10R,13S,14S,17S)-17-ethynyl-17-hydroxy-13-methyl-1, 2,6,7,8,9,10,11,12,14,15, 16-dodecahydrocyclopenta[a]phenanthren-3-one |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 64% |
| Protein binding | >95% |
| Elimination half-life | 7 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C20H26O2 |
| Molar mass | 298.419 g/mol |
Norethisterone (or norethindrone) (or 19-nor-17α-ethynyltestosterone) is a molecule used in some combined oral contraceptive pills and in some progestogen only pills. It is a progestogen and can be used to treat premenstrual syndrome, painful periods, abnormal heavy bleeding, irregular periods, menopausal syndrome (in combination with oestrogen), or to postpone a period. Norethindrone was the first orally highly active progestin to be synthesized. It was synthesized for the first time by chemists Luis E. Miramontes, Carl Djerassi, and George Rosenkranz at Syntex in Mexico City in 1951.[1] It was the progestin used in one of the first two oral contraceptives. It is often used as the related ester, norethisterone acetate.
References
- ↑ Djerassi C, Miramontes L, Rosenkranz G, Sondheimer F (1954). "Steroids. LIV. Synthesis of 19-Nor-17α-ethynyltestosterone and 19-Nor-17α-methyltestosterone" (abstract page). J Am Chem Soc. 76 (16): 4089&ndash, 4091. doi:10.1021/ja01645a009.
Categories:
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Syntex
- Progestagens