Myasthenia gravis pathophysiology
|
Myasthenia gravis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Myasthenia gravis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Myasthenia gravis pathophysiology |
|
Risk calculators and risk factors for Myasthenia gravis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please help WikiDoc by adding more content here. It's easy! Click here to learn about editing.
Pathophysiology
Myasthenia gravis is an autoimmune disease, which features antibodies directed against the body's own proteins. While in various similar diseases the disease has been linked to a cross-reaction with an infective agent, there is no known causative pathogen that could account for myasthenia. There is a slight genetic predisposition, particular HLA types seem to predispose for MG (B8 and DR3 with DR1 more specific for ocular myasthenia). Up to 75% of patients have an abnormality of the thymus; 25% have a thymoma, a tumor (either benign or malignant) of the thymus, and other abnormalities are frequently found. The disease process generally remains stationary after thymectomy (removal of the thymus).
In MG, the autoantibodies are directed most commonly against the acetylcholine receptor (nicotinic type), the receptor in the motor end plate for the neurotransmitter acetylcholine that stimulates muscular contraction. Some forms of the antibody impair the ability of acetylcholine to bind to receptors. Others lead to the destruction of receptors, either by complement fixation or by inducing the muscle cell to eliminate the receptors through endocytosis.
The antibodies are produced by plasma cells, that have been derived from B cells. These plasma cells are activated by T-helper cells, which in turn are activated by binding to acetylcholine receptor antigenic peptide sequences (epitopes) that rest within the histocompatibility antigens of antigen presenting cells. The thymus plays an important role in the development of T-cells, which is why myasthenia gravis is associated with thymoma. The exact mechanism is however not convincingly clarified.
In normal muscle contraction, cumulative activation of the ACh receptor leads to influx of sodium and calcium. Only when the levels of these electrolytes inside the muscle cell is high enough will it contract. Decreased numbers of functioning receptors therefore impairs muscular contraction.
It has recently been realized that a second category of gravis is due to auto-antibodies against the MuSK protein (muscle specific kinase), a tyrosine kinase receptor which is required for the formation of the neuromuscular junction. Antibodies against MuSK inhibit the signaling of MuSK normally induced by its nerve-derived ligand, agrin. The result is a decrease in patency of the neuromuscular junction, and the consequent symptoms of MG.
People treated with penicillamine can develop MG symptoms. Their antibody titer is usually similar to that of MG, but both the symptoms and the titer disappear when drug administration is discontinued.
MG is more common in families with other autoimmune diseases. A familial predisposition is found in 5% of the cases. This is associated with certain genetic variations such as an increased frequency of HLA-B8 and DR3. People with MG suffer from co-existing autoimmune diseases at a higher frequency than members of the general population. Of particular mention is co-existing thyroid disease where episodes of hypothyroidism may precipitate a severe exacerbation.
-
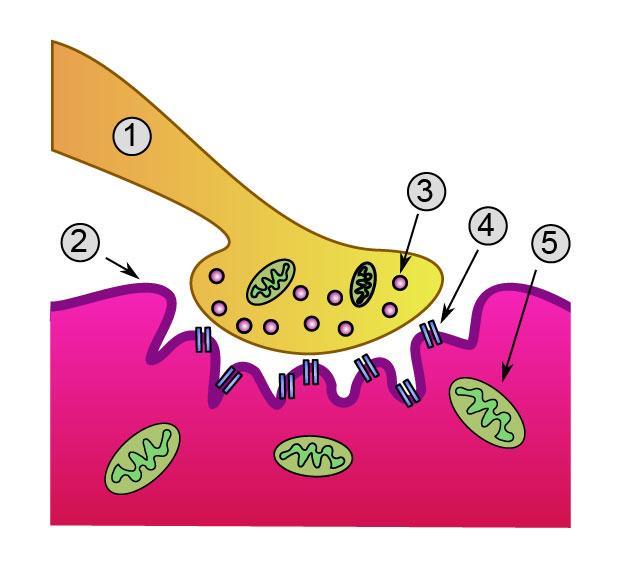
Detailed view of a neuromuscular junction:
1. Presynaptic terminal
2. Sarcolemma
3. Synaptic vesicle
4. Nicotinic acetylcholine receptor
5. Mitochondrion -
Nicotinic Acetylcholine receptor