Melanoma pathophysiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
|
Melanoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Melanoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Melanoma pathophysiology |
|
Risk calculators and risk factors for Melanoma pathophysiology |
Overview
Pathophysiology
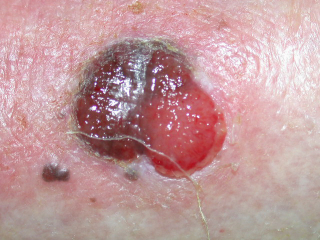
Generally, an individual's risk for developing melanoma depends on two groups of factors: intrinsic and environmental.[1] "Intrinsic" factors are generally an individual's family history and inherited genotype, while the most relevant environmental factor is sun exposure.
Epidemiologic studies suggest that exposure to ultraviolet radiation (UVA[2] and UVB) is one of the major contributors to the development of melanoma. UV radiation causes damage to the DNA of cells, typically thymine dimerization, which when unrepaired can create mutations in the cell's genes. When the cell divides, these mutations are propagated to new generations of cells. If the mutations occur in oncogenes or tumor suppressor genes, the rate of mitosis in the mutation-bearing cells can become uncontrolled, leading to the formation of a tumor. Occasional extreme sun exposure (resulting in "sunburn") is causally related to melanoma.[3] Those with more chronic long term exposure (outdoor workers) may develop protective mechanisms. Melanoma is most common on the back in men and on legs in women (areas of intermittent sun exposure) and is more common in indoor workers than outdoor workers (in a British study[4]). Other factors are mutations in or total loss of tumor suppressor genes. Use of sunbeds (with deeply penetrating UVA rays) has been linked to the development of skin cancers, including melanoma.
Superficial spreading melanoma
Genetics
Familial melanoma is genetically heterogeneous,[5] and loci for familial melanoma have been identified on the chromosome arms 1p, 9p and 12q. Multiple genetic events have been related to the pathogenesis of melanoma.[6] The multiple tumor suppressor 1 (CDKN2A/MTS1) gene encodes p16INK4a - a low-molecular weight protein inhibitor of cyclin-dependent protein kinases (CDKs) - which has been localised to the p21 region of human chromosome 9.[7]
Today, melanomas are diagnosed only after they become visible on the skin. In the future, however, physicians will hopefully be able detect melanomas based on a patient’s genotype, not just his or her phenotype. Recent genetic advances promise to help doctors to identify people with high-risk genotypes and to determine which of a person’s lesions have the greatest chance of becoming cancerous.
A number of rare mutations, which often run in families, are known to greatly increase one’s susceptibility to melanoma. One class of mutations affects the gene CDKN2A. An alternative reading frame mutation in this gene leads to the destabilization of p53, a transcription factor involved in apoptosis and in fifty percent of human cancers. Another mutation in the same gene results in a non-functional inhibitor of CDK4, a [cyclin-dependent kinase] that promotes cell division. Mutations that cause the skin condition Xeroderma Pigmentosum (XP) also seriously predispose one to melanoma. Scattered throughout the genome, these mutations reduce a cell’s ability to repair DNA. Both CDKN2A and XP mutations are highly penetrant.
Other mutations confer lower risk but are more prevalent in the population. People with mutations in the MC1R gene, for example, are two to four times more likely to develop melanoma than those with two wild-type copies of the gene. MC1R mutations are very common; in fact, all people with red hair have a mutated copy of the gene. Two-gene models of melanoma risk have already been created, and in the future, researchers hope to create genome-scale models that will allow them to predict a patient’s risk of developing melanoma based on his or her genotype.
Pathology
| Common Subclasses | Features on Gross Pathology | Features on Histopathological Microscopic Analysis |
| Superficial spreading melanoma |
|
|
| Nodular melanoma |
|
|
| Acral lentiginous melanoma |
|
|
| Lentigo maligna melanoma |
|
|
| Non-cutaneous melanoma |
|
|
| Dermoplastic/Spindle cell melanoma |
|
|
| Nevoid melanoma |
|
|
| Spitzoid melanocytic neoplasm |
|
|
| Angiotropic melanoma |
|
|
| Blue nevus-like melanoma |
|
|
| Composite melanoma |
Features of more than one subtype on gross pathology |
|
References
- ↑ Who is Most at Risk for Melanoma?
- ↑ Wang S, Setlow R, Berwick M, Polsky D, Marghoob A, Kopf A, Bart R (2001). "Ultraviolet A and melanoma: a review". J Am Acad Dermatol. 44 (5): 837–46. PMID 11312434.
- ↑ Oliveria S, Saraiya M, Geller A, Heneghan M, Jorgensen C (2006). "Sun exposure and risk of melanoma". Arch Dis Child. 91 (2): 131–8. PMID 16326797.
- ↑ Lee J, Strickland D (1980). "Malignant melanoma: social status and outdoor work". Br J Cancer. 41 (5): 757–63. PMID 7426301.
- ↑ Greene MH. (1998). "The genetics of hereditary melanoma and nevi". Cancer. 86 (11): 2464–2477. PMID 10630172.
- ↑ Halachmi S, Gilchrest BA. (2001). "Update on genetic events in the pathogenesis of melanoma". Curr Opin Oncol. 13 (2): 129–136. PMID 11224711.
- ↑ CDKN2A cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) from Entrez Gene