Malaria pathophysiology
For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
|
Malaria Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Malaria pathophysiology On the Web |
|
American Roentgen Ray Society Images of Malaria pathophysiology |
|
Risk calculators and risk factors for Malaria pathophysiology |
Overview
Malaria is a vector-borne infectious disease caused by protozoan parasites. It is widespread in tropical and subtropical regions, including parts of the Americas, Asia, and Africa. Each year, it causes disease in approximately 650 million people and kills between one and three million, most of them young children in Sub-Saharan Africa. Malaria is commonly associated with poverty, but is also a cause of poverty and a major hindrance to economic development.
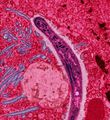
Pathophysiology

Malaria in humans develops via two phases: an exoerythrocytic (hepatic) and an erythrocytic phase. When an infected mosquito pierces a person's skin to take a blood meal, sporozoites in the mosquito's saliva enter the bloodstream and migrate to the liver. Within 30 minutes of being introduced into the human host, they infect hepatocytes, multiplying asexually and asymptomatically for a period of 6–15 days. Once in the liver these organisms differentiate to yield thousands of merozoites which, following rupture of their host cells, escape into the blood and infect red blood cells, thus beginning the erythrocytic stage of the life cycle.[1] The parasite escapes from the liver undetected by wrapping itself in the cell membrane of the infected host liver cell.[2]
Within the red blood cells the parasites multiply further, again asexually, periodically breaking out of their hosts to invade fresh red blood cells. Several such amplification cycles occur. Thus, classical descriptions of waves of fever arise from simultaneous waves of merozoites escaping and infecting red blood cells.
Some P. vivax and P. ovale sporozoites do not immediately develop into exoerythrocytic-phase merozoites, but instead produce hypnozoites that remain dormant for periods ranging from several months (6–12 months is typical) to as long as three years. After a period of dormancy, they reactivate and produce merozoites. Hypnozoites are responsible for long incubation and late relapses in these two species of malaria.[3]
The parasite is relatively protected from attack by the body's immune system because for most of its human life cycle it resides within the liver and blood cells and is relatively invisible to immune surveillance. However, circulating infected blood cells are destroyed in the spleen. To avoid this fate, the P. falciparum parasite displays adhesive proteins on the surface of the infected blood cells, causing the blood cells to stick to the walls of small blood vessels, thereby sequestering the parasite from passage through the general circulation and the spleen.[4] This "stickiness" is the main factor giving rise to hemorrhagic complications of malaria. High endothelial venules (the smallest branches of the circulatory system) can be blocked by the attachment of masses of these infected red blood cells. The blockage of these vessels causes symptoms such as in placental and cerebral malaria. In cerebral malaria the sequestrated red blood cells can breach the blood brain barrier possibly leading to coma.[5]
Although the red blood cell surface adhesive proteins (called PfEMP1, for Plasmodium falciparum erythrocyte membrane protein 1) are exposed to the immune system they do not serve as good immune targets because of their extreme diversity; there are at least 60 variations of the protein within a single parasite and perhaps limitless versions within parasite populations.[4] Like a thief changing disguises or a spy with multiple passports, the parasite switches between a broad repertoire of PfEMP1 surface proteins, thus staying one step ahead of the pursuing immune system.
Some merozoites turn into male and female gametocytes. If a mosquito pierces the skin of an infected person, it potentially picks up gametocytes within the blood. Fertilization and sexual recombination of the parasite occurs in the mosquito's gut, thereby defining the mosquito as the definitive host of the disease. New sporozoites develop and travel to the mosquito's salivary gland, completing the cycle. Pregnant women are especially attractive to the mosquitoes,[6] and malaria in pregnant women is an important cause of stillbirths, infant mortality and low birth weight.[7]
References
- ↑ Bledsoe, G. H. (December 2005) "Malaria primer for clinicians in the United States" Southern Medical Journal 98(12): pp. 1197-204, (PMID: 16440920);
- ↑ Sturm A,
Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT (2006). "Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids". Science. 313: 1287–1490. PMID 16888102. line feed character in
|author=at position 10 (help) - ↑ Cogswell F (1992). "The hypnozoite and relapse in primate malaria". Clin Microbiol Rev. 5 (1): 26–35. PMID 1735093.
- ↑ 4.0 4.1 Chen Q, Schlichtherle M, Wahlgren M (2000). "Molecular aspects of severe malaria". Clin Microbiol Rev. 13 (3): 439–50. PMID 10885986.
- ↑ Adams S, Brown H, Turner G (2002). "Breaking down the blood-brain barrier: signaling a path to cerebral malaria?". Trends Parasitol. 18 (8): 360–6. PMID 12377286.
- ↑ Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G (2000). "Effect of pregnancy on exposure to malaria mosquitoes". Lancet. 355 (9219): 1972. PMID 10859048.
- ↑ van Geertruyden J, Thomas F, Erhart A, D'Alessandro U (2004). "The contribution of malaria in pregnancy to perinatal mortality". Am J Trop Med Hyg. 71 (2 Suppl): 35–40. PMID 15331817.