Langerhans cell histiocytosis pathophysiology
|
Langerhans cell histiocytosis Microchapters |
|
Differentiating Langerhans cell histiocytosis from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Langerhans cell histiocytosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Langerhans cell histiocytosis pathophysiology |
|
Directions to Hospitals Treating Langerhans cell histiocytosis |
|
Risk calculators and risk factors for Langerhans cell histiocytosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Haytham Allaham, M.D. [2]
Overview
Langerhans cell histiocytosis arises from epidermal dendritic cells, which are normally involved in the process of antigen presentation to lymphocytic cells. Langerhans cells originally arise from the bone marrow, then the cells migrate to other organs such as the skin, lymph nodes, and lungs. The exact pathogenesis of Langerhans cell histiocytosis is not fully understood. It is thought that Langerhans cell histiocytosis is the result of either a true neoplastic process or a reactive immune condition. Genes commonly involved in the pathogenesis of Langerhans cell histiocytosis include BRAF gene and MAP2K1 gene. On gross pathology, scaly erythema, red papules, and extensive eruptions located on the scalp are characteristic finding of Langerhans cell histiocytosis. Characteristic findings of Langerhans cell histiocytosis on light microscopy may include clusters of dendritic cells with kidney-shaped nucleus and abundant foamy cytoplasm. On electron microscopy Langerhans cell histiocytosis is characterized by Birbeck granules, which are electron dense, cytoplasmic, tennis racket-like bodies. On immunohistochemistry Langerhans cell histiocytosis is characterized by positivity to CD1a, S100, and CD207 (langerin).
Pathogenesis
- Langerhans cell histiocytosis arises from epidermal dendritic cells, which are normally involved in the process of antigen presentation to lymphocytic cells.
- Langerhans cells originally arise from the bone marrow, then the cells migrate to other organs such as:
- The malignant organ involvement is variable among patients with Langerhans cell histiocytosis. The disease process may range from an isolated cutaneous or bone involvement to a life-threatening multi-system condition.
- The skeletal distribution of Langerhans cell histiocytosis involves the following sites:
- The skull (most common bone involved)
- Ribs
- Humerus
- Vertebra
- The exact pathogenesis of Langerhans cell histiocytosis is not fully understood. It is thought that Langerhans cell histiocytosis is the result of either a true neoplastic process or a reactive immune condition.
- Facts consistent with the neoplastic process hypothesis include the following:
- The infiltration of organs by monoclonal population of pathologic cells
- The presence of specific recurrent cytogenetic and genomic abnormalities
- The successful treatment of a subset of disseminated Langerhans cell histiocytosis using chemotherapeutic regimens
- Facts consistent with the reactive immune condition hypothesis include the following:
- The evidence of spontaneous remissions that may occur among certain cases of Langerhans cell histiocytosis
- The extensive secretion of multiple cytokines by dendritic cells and bystander-cells (a phenomenon known as cytokine storm)
- The favorable prognosis and relatively good survival rate among patients with no organ dysfunction
- The uncontrolled monoclonal proliferation of Langerhans cells will initiate a non-specific inflammatory response, which will lead to the accumulation of various immune system cells such as:
- Eosinophils
- Macrophages
- Lymphocytes
- Multinucleated giant cells
- Langerhans cell histiocytosis may result in bone marrow failure due to malignant cell infiltration of the bone marrow. This can manifest as:
- Anemia
- Recurrent bleeding
- Recurrent Infections
- The infiltration of the hypothalamic pituitary axis by malignant Langerhans cells will lead to a deficiency in both anterior and posterior pituitary hormones, which may result in:
- Central diabetes insipidus
- Growth retardation
- Delayed puberty
- Langerhans cell histiocytosis may also infiltrate the lymph nodes, spleen, and liver.
Genetics
- Development of Langerhans cell histiocytosis is the result of multiple genetic mutations.
- Genes commonly involved in the pathogenesis of Langerhans cell histiocytosis include:
- BRAF gene mutation located on Chromosome 7
- MAP2K1 gene mutation located on Chromosome 15
Associated Conditions
- Langerhans cell histiocytosis is associated with a number of syndromes that include:
- Hand-Schüller-Christian triad
- Hashimoto-Pritzker disease
- Letterer-Siwe disease
Gross Pathology
- On gross pathology, scaly erythematous, red papules, and extensive eruptions located on the scalp are characteristic finding of Langerhans cell histiocytosis.
- The image below demonstrates the cutaneous lesions observed among Langerhans cell histiocytosis patients:
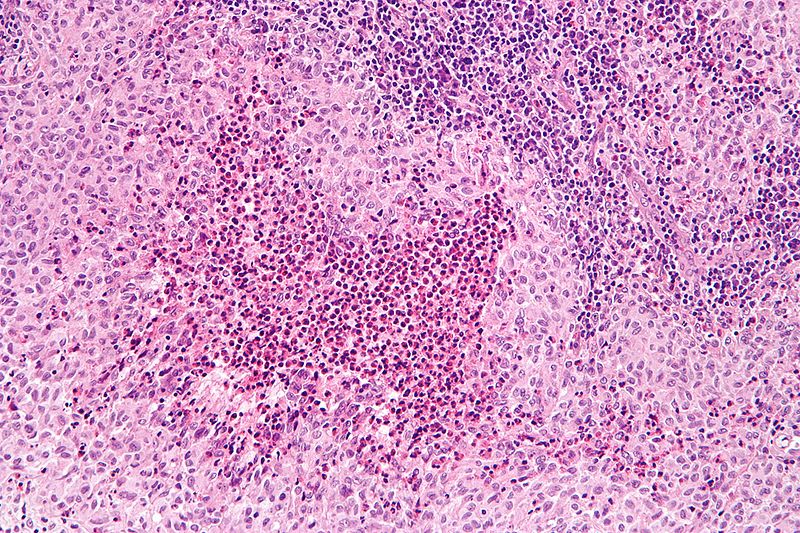
Microscopic Pathology
- Characteristic findings of Langerhans cell histiocytosis on light microscopy may include:
- Clusters of dendritic cells
- Kidney-shaped nucleus
- Abundant foamy cytoplasm
- Fine, granular chromatin pattern
- Prominent eosinophils
- Fibrosis
- Other inflammatory cells may be present (neutrophils, plasma cells, and multinucleated giant cells)
- On electron microscopy Langerhans cell histiocytosis is characterized by Birbeck granules, which are electron dense, cytoplasmic, tennis racket-like bodies.
- On immunohistochemistry Langerhans cell histiocytosis is characterized by:
- CD1a +ve
- S100 +ve
- CD207 (langerin) +ve
Gallery
Illustrated below is a series of microscopic images demonstrating Langerhans cell histiocytosis: