Granulocytic sarcoma: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
{{SK}} Chloroma, Myeloid Sarcoma, Myeloid Sarcomas, Myeloid Cell Tumor, Granulocytic Sarcoma, Extramedullary Myeloid Cell Tumor | {{SK}} Chloroma, Myeloid Sarcoma, Myeloid Sarcomas, Myeloid Cell Tumor, Granulocytic Sarcoma, Extramedullary Myeloid Cell Tumor | ||
==Overview== | ==Overview== | ||
Granulocytic sarcoma was first described by the British [[physician]] A. Burns in 1811. A granulocytic sarcoma is a solid [[tumor]] composed of immature [[malignant]] [[white blood cell]]s called [[myeloblast]]s. Grossly the neoplastic tissue usually appears firm with a fish-flesh appearance. On microscopic histopathological analysis, diffuse monotonous infiltrate that may or may not destroy underlying normal structures, tingible body macrophages | Granulocytic sarcoma was first described by the British [[physician]] A. Burns in 1811. A granulocytic sarcoma is a solid [[tumor]] composed of immature [[malignant]] [[white blood cell]]s called [[myeloblast]]s. Grossly the neoplastic tissue usually appears firm with a fish-flesh appearance. On microscopic histopathological analysis, diffuse monotonous infiltrate that may or may not destroy underlying normal structures, tingible body macrophages that impart a starry sky appearance are characteristic findings of granulocytic sarcoma. Granulocytic sarcoma must be differentiated from other diseases that cause lymphoma such as non-Hodgkin lymphomas of the lymhoblastic type, [[Burkitt lymphoma]], large-cell lymphoma, and small round cell tumors. Symptoms of granulocytic sarcoma may include violaceous, raised, nontender plaques or nodules on skin and painful gums which bleed easily with tooth brushing and other minor trauma. Physical examination may be remarkable for gingival hypertrophyt, lymphadenopathy, and violaceous, raised, nontender plaques or nodules on skin. Findings on immunohistochemical stainings include CD20, CD43, CD68, and myeloperoxidase. Systemic [[chemotherapy]] against the leukemia is typically utilized as the first-line treatment. | ||
==Historical Perspective== | ==Historical Perspective== | ||
* Granulocytic sarcoma was first described by the British [[physician]] A. Burns in 1811<ref>Burns A. Observations of surgical anatomy, in Head and Neck. London, England, Royce, 1811, p. 364.</ref>, although the term ''chloroma'' did not appear until 1853.<ref>King A. A case of chloroma. Monthly J Med 17:17, 1853.</ref> This name is derived from the [[Greek language|Greek]] word ''chloros'' (green), as these tumors often have a green tint due to the presence of [[myeloperoxidase]]. The link between chloroma and [[acute leukemia]] was first recognized in 1902 by Dock and Warthin.<ref>Dock G, Warthin AS. A new case of chloroma with leukemia. Trans Assoc Am Phys 19:64, 1904, p. 115.</ref> However, because up to 30% of these tumors can be white, gray, or brown rather than green, the more correct term ''granulocytic sarcoma'' was proposed by Rappaport in 1967<ref>Rappaport H. Tumors of the hematopoietic system, in Atlas of Tumor Pathology, Section III, Fascicle 8. Armed Forces Institute of Pathology, Washington DC, 1967, pp. 241-247.</ref> and has since become virtually synonymous with the term chloroma. | * Granulocytic sarcoma was first described by the British [[physician]] A. Burns in 1811<ref>Burns A. Observations of surgical anatomy, in Head and Neck. London, England, Royce, 1811, p. 364.</ref>, although the term ''chloroma'' did not appear until 1853.<ref>King A. A case of chloroma. Monthly J Med 17:17, 1853.</ref> This name is derived from the [[Greek language|Greek]] word ''chloros'' (green), as these tumors often have a green tint due to the presence of [[myeloperoxidase]]. The link between chloroma and [[acute leukemia]] was first recognized in 1902 by Dock and Warthin.<ref>Dock G, Warthin AS. A new case of chloroma with leukemia. Trans Assoc Am Phys 19:64, 1904, p. 115.</ref> However, because up to 30% of these tumors can be white, gray, or brown rather than green, the more correct term ''granulocytic sarcoma'' was proposed by Rappaport in 1967<ref>Rappaport H. Tumors of the hematopoietic system, in Atlas of Tumor Pathology, Section III, Fascicle 8. Armed Forces Institute of Pathology, Washington DC, 1967, pp. 241-247.</ref> and has since become virtually synonymous with the term chloroma. | ||
| Line 30: | Line 30: | ||
* Very rarely, chloroma can occur without a known pre-existing or concomitant diagnosis of acute leukemia or MDS/MPS; this is known as primary chloroma. Diagnosis is particularly challenging in this situation (see below). In almost all reported cases of primary chloroma, acute leukemia has developed shortly afterward (median time to development of acute leukemia 7 months, range 1-25 months). Therefore, primary chloroma should probably be considered an initial manifestation of acute leukemia, rather than a localized process, and treated as such. | * Very rarely, chloroma can occur without a known pre-existing or concomitant diagnosis of acute leukemia or MDS/MPS; this is known as primary chloroma. Diagnosis is particularly challenging in this situation (see below). In almost all reported cases of primary chloroma, acute leukemia has developed shortly afterward (median time to development of acute leukemia 7 months, range 1-25 months). Therefore, primary chloroma should probably be considered an initial manifestation of acute leukemia, rather than a localized process, and treated as such. | ||
* Grossly the neoplastic tissue usually appears firm with a fish-flesh appearance. | * Grossly the neoplastic tissue usually appears firm with a fish-flesh appearance. | ||
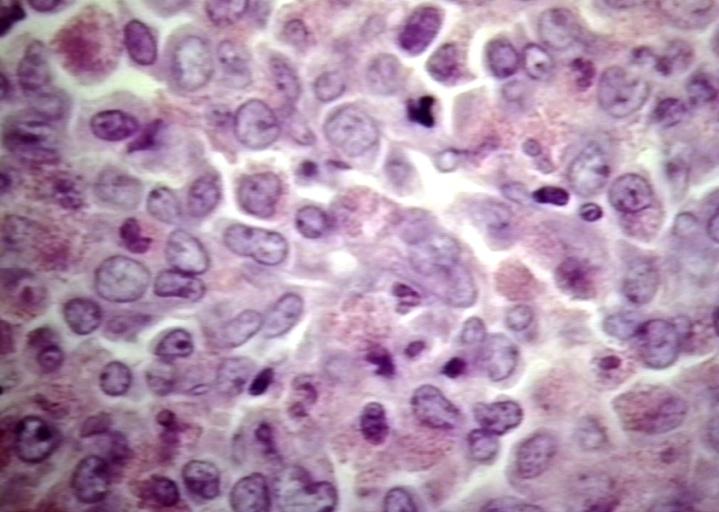
*On microscopic histopathological analysis, diffuse monotonous infiltrate that may or may not destroy underlying normal structures, tingible body macrophages | *On microscopic histopathological analysis, diffuse monotonous infiltrate that may or may not destroy underlying normal structures, tingible body macrophages that impart a starry sky appearance are characteristic findings of granulocytic sarcoma. | ||
[[Image:Granulocytic sarcoma.jpg|500px|thumb|left|Lymph node: Granulocytic sarcoma. [http://www.peir.net Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology]]] | [[Image:Granulocytic sarcoma.jpg|500px|thumb|left|Lymph node: Granulocytic sarcoma. [http://www.peir.net Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology]]] | ||
<br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/> | <br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/> | ||
Revision as of 16:59, 13 May 2016
For patient information, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Synonyms and keywords: Chloroma, Myeloid Sarcoma, Myeloid Sarcomas, Myeloid Cell Tumor, Granulocytic Sarcoma, Extramedullary Myeloid Cell Tumor
Overview
Granulocytic sarcoma was first described by the British physician A. Burns in 1811. A granulocytic sarcoma is a solid tumor composed of immature malignant white blood cells called myeloblasts. Grossly the neoplastic tissue usually appears firm with a fish-flesh appearance. On microscopic histopathological analysis, diffuse monotonous infiltrate that may or may not destroy underlying normal structures, tingible body macrophages that impart a starry sky appearance are characteristic findings of granulocytic sarcoma. Granulocytic sarcoma must be differentiated from other diseases that cause lymphoma such as non-Hodgkin lymphomas of the lymhoblastic type, Burkitt lymphoma, large-cell lymphoma, and small round cell tumors. Symptoms of granulocytic sarcoma may include violaceous, raised, nontender plaques or nodules on skin and painful gums which bleed easily with tooth brushing and other minor trauma. Physical examination may be remarkable for gingival hypertrophyt, lymphadenopathy, and violaceous, raised, nontender plaques or nodules on skin. Findings on immunohistochemical stainings include CD20, CD43, CD68, and myeloperoxidase. Systemic chemotherapy against the leukemia is typically utilized as the first-line treatment.
Historical Perspective
- Granulocytic sarcoma was first described by the British physician A. Burns in 1811[1], although the term chloroma did not appear until 1853.[2] This name is derived from the Greek word chloros (green), as these tumors often have a green tint due to the presence of myeloperoxidase. The link between chloroma and acute leukemia was first recognized in 1902 by Dock and Warthin.[3] However, because up to 30% of these tumors can be white, gray, or brown rather than green, the more correct term granulocytic sarcoma was proposed by Rappaport in 1967[4] and has since become virtually synonymous with the term chloroma.
- The term chloroma was introduced by King in 1853 because of the green color of the lesion when exposed to air.
Pathophysiology
- A granulocytic sarcoma is a solid tumor composed of immature malignant white blood cells called myeloblasts. A chloroma is an extramedullary manifestion of acute myeloid leukemia
- Currently, any extramedullary manifestion of acute myeloid leukemia can be termed a granulocytic sarcoma or chloroma.
- Specific terms which overlap with granulocytic sarcoma include:
- Leukemia cutis, describing infiltration of the dermis (skin) by leukemic cells, which is also referred to as cutaneous granulocytic sarcoma
- Meningeal leukemia, or invasion of the subarachnoid space by leukemic cells, is usually considered distinct from chloroma, although very rarely occurring solid central nervous system tumors composed of leukemic cells can be termed chloromas.
In acute leukemia
- Chloromas are rare; exact estimates of their incidence are lacking, but they are uncommonly seen even by physicians specializing in the treatment of leukemia. Chloromas may be somewhat more common in patients with the following disease features:[5]
- FAB class M4 or M5
- Those with specific cytogenetic abnormalities (e.g. t(8;21) or inv(16))
- Those whose myeloblasts express T-cell surface markers, CD13, or CD14
- Those with high peripheral white blood cell counts
- However, even in patients with the above risk factors, chloroma remains an uncommon complication of acute myeloid leukemia.
- Rarely, a chloroma can develop as the sole manifestation of relapse after apparently successful treatment of acute myeloid leukemia. In keeping with the general behavior of chloromas, such an event must be regarded as an early herald of a systemic relapse, rather than as a localized process. In one review of 24 patients who developed isolated chloromas after treatment for acute myeloid leukemia, the mean interval until bone marrow relapse was 7 months (range, 1 to 19 months).[6]
- The most common localizations are skin, soft tissue, bone, and lymph nodes.1 Primary involvement of the gastrointestinal (GI) tract is rare.
In myeloproliferative or myelodysplastic syndromes
- Chloromas may occur in patients with a diagnosis of myelodysplastic syndrome (MDS) or myeloproliferative syndromes (MPS) (e.g. chronic myelogenous leukemia (CML), polycythemia vera, essential thrombocytosis, or myelofibrosis). The detection of a chloroma is considered de facto evidence that these pre-malignant conditions have transformed into an acute leukemia requiring appropriate treatment. For example, presence of a chloroma is sufficient to indicate that chronic myelogenous leukemia has entered its blast crisis phase.
Primary chloroma
- Very rarely, chloroma can occur without a known pre-existing or concomitant diagnosis of acute leukemia or MDS/MPS; this is known as primary chloroma. Diagnosis is particularly challenging in this situation (see below). In almost all reported cases of primary chloroma, acute leukemia has developed shortly afterward (median time to development of acute leukemia 7 months, range 1-25 months). Therefore, primary chloroma should probably be considered an initial manifestation of acute leukemia, rather than a localized process, and treated as such.
- Grossly the neoplastic tissue usually appears firm with a fish-flesh appearance.
- On microscopic histopathological analysis, diffuse monotonous infiltrate that may or may not destroy underlying normal structures, tingible body macrophages that impart a starry sky appearance are characteristic findings of granulocytic sarcoma.
Differentiating Granulocytic sarcoma from other Diseases
- Granulocytic sarcoma must be differentiated from other diseases that cause lymphoma such as:
- Non-Hodgkin lymphomas of the lymhoblastic type
- Burkitt lymphoma
- Large-cell lymphoma
- Small round cell tumors
Epidemiology and Demographics
Age
- Granulocytic sarcoma is more commonly observed among older patients with a median age of 56 years.
Gender
- Males are more commonly affected with granulocytic sarcoma than females with male-to-female ratio of 1:2.
Natural History, Complications and Prognosis
- There is conflicting evidence on the prognostic significance of chloromas in patients with acute myeloid leukemia. In general, they are felt to augur a poorer prognosis, with a poorer response to treatment and worse survival[7]; however, others have reported that chloromas associate, as a biologic marker, with other poor prognostic factors, and therefore do not have independent prognostic significance.[8]
Diagnosis
Symptoms
- Symptoms of granulocytic sarcoma may include the following:
- Violaceous, raised, nontender plaques or nodules on skin
- Painful gums which bleed easily with tooth brushing and other minor trauma
Physical Examination
- Physical examination may be remarkable for:
- Gingival hypertrophy
- Lymphadenopathy
- Violaceous, raised, nontender plaques or nodules on skin
Laboratory Findings
- There are no specific laboratory findings associated with granulocytic sarcoma.
Imaging Findings=
- There are no imaging study findings associated with granulocytic sarcoma.
Other Diagnostic Studies
- Granulocytic sarcoma may also be diagnosed using immunohistochemical stainings for expression of myeloid associated enzymes.
- Findings on immunohistochemical stainings include CD20, CD43, CD68, and myeloperoxidase.
- Definitive diagnosis of a chloroma usually requires a biopsy of the lesion in question.
Treatment
Medical Therapy
- Systemic chemotherapy against the leukemia is typically utilized as the first-line treatment, unless there is an emergent indication for local treatment of the chloroma (e.g. compromise of the spinal cord). Chloromas are typically quite sensitive to standard anti-leukemic chemotherapy.
- If the chloroma is persistent after completion of induction chemotherapy, local treatment such as surgery or radiation therapy is often considered.
- Patients presenting with a primary chloroma typically receive systemic chemotherapy, as development of acute leukemia is nearly universal in the short term after detection of the chloroma.
- Patients treated for acute leukemia who relapse with an isolated chloroma are typically treated with systemic therapy for relapsed leukemia. However, as with any relapsed leukemia, outcomes are unfortunately poor.
- Patients with "pre-leukemic" conditions such as myelodysplastic syndromes or myeloproliferative syndromes who develop a chloroma are often treated as if they have transformed to acute leukemia.
Prevention
- There are no primary preventive measures available for granulocytic sarcoma.
References
- ↑ Burns A. Observations of surgical anatomy, in Head and Neck. London, England, Royce, 1811, p. 364.
- ↑ King A. A case of chloroma. Monthly J Med 17:17, 1853.
- ↑ Dock G, Warthin AS. A new case of chloroma with leukemia. Trans Assoc Am Phys 19:64, 1904, p. 115.
- ↑ Rappaport H. Tumors of the hematopoietic system, in Atlas of Tumor Pathology, Section III, Fascicle 8. Armed Forces Institute of Pathology, Washington DC, 1967, pp. 241-247.
- ↑ Byrd JC, Edenfield JW, Shields DJ, et al: Extramedullary myeloid tumours in acute nonlymphocytic leukaemia: A clinical review. J Clin Oncol 13:1800, 1995.
- ↑ Byrd JC, Weiss RB. Recurrent granulocytic sarcoma: an unusual variation of acute myeloid leukemia associated with 8;21 chromosomal translocation and blast expression of the neural cell adhesion molecule. Cancer 73:2107-2112, 1994.
- ↑ Tanravahi R, Qumsiyeh M, Patil S, et al: Extramedullary leukemia adversely affects hematologic complete remission and overall survival in patients with t(8;21)(q22;q22): Results from Cancer and Leukemia Group B 8461. J Clin Oncol 15:466, 1997.
- ↑ Bisschop MM, Revesz T, Bierings M, et al: Extramedullary infiltrates at diagnosis have no prognostic significance in children with acute myeloid leukemia. Leukemia 15:46, 2001.