Glycerol phenylbutyrate: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 66: | Line 66: | ||
* Oral liquid: colorless to pale yellow, 1.1 g/mL of glycerol phenylbutyrate (delivers 1.02 g/mL of phenylbutyrate). | * Oral liquid: colorless to pale yellow, 1.1 g/mL of glycerol phenylbutyrate (delivers 1.02 g/mL of phenylbutyrate). | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 79: | Line 77: | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|contraindications='''RAVICTI is contraindicated in patients''' | |contraindications='''RAVICTI is contraindicated in patients''' | ||
| Line 117: | Line 111: | ||
* Adverse reactions occurring in ≥10% of pediatric patients were upper [[abdominal pain]], [[rash]], [[nausea]], [[vomiting]], [[diarrhea]], decreased appetite, [[hyperammonemia]], and [[headache]]. | * Adverse reactions occurring in ≥10% of pediatric patients were upper [[abdominal pain]], [[rash]], [[nausea]], [[vomiting]], [[diarrhea]], decreased appetite, [[hyperammonemia]], and [[headache]]. | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
|drugInteractions='''Potential for Other Drugs to Affect Ammonia''' | |drugInteractions='''Potential for Other Drugs to Affect Ammonia''' | ||
| Line 139: | Line 127: | ||
* Probenecid may inhibit the renal excretion of metabolites of RAVICTI including PAGN and PAA. | * Probenecid may inhibit the renal excretion of metabolites of RAVICTI including PAGN and PAA. | ||
|useInPregnancyFDA= | |useInPregnancyFDA='''Pregnancy Category C''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ''Risk Summary'' | ||
* There are no adequate and well-controlled studies in pregnant women. In rabbits given glycerol phenylbutyrate at doses up to 2.7 times the dose of 6.87 mL/m2/day in adult patients (based on combined area under the curve [AUCs] for PBA and PAA) during the period of organogenesis, maternal toxicity, but no effects on embryo-fetal development, was observed. In rats given glycerol phenylbutyrate at 1.9 times the dose of 6.87 mL/m2/day in adult patients (based on combined AUCs for PBA and PAA), no adverse embryo-fetal effects were observed. Maternal toxicity, reduced fetal weights, and variations in skeletal development were observed in rats at doses greater than or equal to 5.7 times the dose of 6.87 mL/m2/day in adult patients (based on combined AUCs for PBA and PAA). RAVICTI should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
''Animal Data'' | |||
* Oral administration of glycerol phenylbutyrate during the period of organogenesis up to 350 mg/kg/day in rabbits produced maternal toxicity, but no effects on embryo-fetal development. The dose of 350 mg/kg/day in rabbits is approximately 2.7 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA. In rats, at an oral dose of 300 mg/kg/day of glycerol phenylbutyrate (1.9 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) during the period of organogenesis, no effects on embryo-fetal development were observed. Doses ≥650 mg/kg/day produced maternal toxicity and adverse effects on embryo-fetal development including reduced fetal weights and cervical ribs at the 7th cervical vertebra. The dose of 650 mg/kg/day in rats is approximately 5.7 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA. No developmental abnormalities, effects on growth, or effects on learning and memory were observed in rats through day 92 postpartum following oral administration in pregnant rats with up to 900 mg/kg/day of glycerol phenylbutyrate (8.5 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) during organogenesis and lactation. | |||
|useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing=* It is not known whether RAVICTI or its metabolites are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions from RAVICTI in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into consideration the importance of the drug to the health of the mother. | ||
|useInPed= | |useInPed='''Patients Between 2 and <18 Years of Age''' | ||
|useInGeri= | |||
* The safety and efficacy of RAVICTI in patients 2 to <18 years of age were established in 2 open-label, sodium phenylbutyrate to RAVICTI, fixed-sequence, switchover clinical trials. | |||
'''Patients ≥2 Months and <2 Years of Age''' | |||
* The safety and efficacy of RAVICTI in patients 2 months to <2 years of age has not been established. PK and ammonia control were studied in only 4 patients between 2 months and <2 years of age, providing insufficient data to establish a safe and effective dose in this age range. | |||
'''Patients <2 Months of Age''' | |||
* RAVICTI is contraindicated in patients <2 months of age. Children <2 months of age may have immature pancreatic exocrine function, which could impair hydrolysis of RAVICTI. Pancreatic lipases may be necessary for intestinal hydrolysis of RAVICTI, allowing release of phenylbutyrate and subsequent formation of PAA, the active moiety. It is not known whether pancreatic and extrapancreatic lipases are sufficient for hydrolysis of RAVICTI. If there is inadequate intestinal hydrolysis of RAVICTI, impaired absorption of phenylbutyrate and hyperammonemia could occur. | |||
'''Juvenile Animal Study''' | |||
* In a juvenile rat study with daily oral dosing performed on postpartum day 2 through mating and pregnancy after maturation, terminal body weight was dose-dependently reduced by up to 16% in males and 12% in females. Learning, memory, and motor activity endpoints were not affected. However, fertility (number of pregnant rats) was decreased by up to 25% at ≥650 mg/kg/day (2.6 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA). Embryo toxicity (increased resorptions) occurred at 650 mg/kg/day (2.6 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) and litter size was reduced at 900 mg/kg/day (3 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA). | |||
|useInGeri=* Clinical studies of RAVICTI did not include sufficient numbers of subjects ≥65 years of age to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair= | |useInRenalImpair=* The efficacy and safety of RAVICTI in patients with renal impairment are unknown. Monitor ammonia levels closely when starting patients with impaired renal function on RAVICTI. | ||
|useInHepaticImpair= | |useInHepaticImpair=* No studies were conducted in UCD patients with hepatic impairment. Because conversion of PAA to PAGN occurs in the liver, patients with hepatic impairment may have reduced conversion capability and higher plasma PAA and PAA to PAGN ratio. Therefore, dosage for patients with moderate to severe hepatic impairment should be started at the lower end of the recommended dosing range and should be kept on the lowest dose necessary to control their ammonia levels. | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
|administration=* Oral | |administration=* Oral | ||
| Line 172: | Line 178: | ||
:*Flush a second time with an additional 30 mL of water to clear the tube. | :*Flush a second time with an additional 30 mL of water to clear the tube. | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
| Line 184: | Line 188: | ||
|overdose=* While there is no experience with overdosage in human clinical trials, PAA, a toxic metabolite of RAVICTI, can accumulate in patients who receive an overdose. In case of overdosage, discontinue the drug and contact poison control. | |overdose=* While there is no experience with overdosage in human clinical trials, PAA, a toxic metabolite of RAVICTI, can accumulate in patients who receive an overdose. In case of overdosage, discontinue the drug and contact poison control. | ||
|drugBox=<!--Mechanism of Action--> | |drugBox=<!--Mechanism of Action--> | ||
|mechAction=* | |mechAction=* UCDs are inherited deficiencies of enzymes or transporters necessary for the synthesis of urea from ammonia (NH3, NH4+). Absence of these enzymes or transporters results in the accumulation of toxic levels of ammonia in the blood and brain of affected patients. RAVICTI is a triglyceride containing 3 molecules of phenylbutyrate (PBA). PAA, the major metabolite of PBA, is the active moiety of RAVICTI. PAA conjugates with glutamine (which contains 2 molecules of nitrogen) via acetylation in the liver and kidneys to form PAGN, which is excreted by the kidneys (Figure 1). On a molar basis, PAGN, like urea, contains 2 moles of nitrogen and provides an alternate vehicle for waste nitrogen excretion. | ||
[[File:Ravicite mechanism of action.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|structure=* RAVICTI (glycerol phenylbutyrate) is a clear, colorless to pale yellow oral liquid. It is insoluble in water and most organic solvents, and it is soluble in dimethylsulfoxide (DMSO) and >65% acetonitrile. | |||
* Glycerol phenylbutyrate is a nitrogen-binding agent. It is a triglyceride containing 3 molecules of PBA linked to a glycerol backbone, the chemical name of which is benzenebutanoic acid, 1', 1' ' –(1,2,3-propanetriyl) ester with a molecular weight of 530.67. It has a molecular formula of C33H38O6. The structural formula is: | |||
[[File:Raviciti structure.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
Revision as of 14:15, 28 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Glycerol phenylbutyrate is a {{{drugClass}}} that is FDA approved for the treatment of patients with urea cycle disorders (UCDs) that cannot be managed by dietary protein restriction and/or amino acid supplementation alone.. Common adverse reactions include diarrhea, flatulence and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- RAVICTI is indicated for use as a nitrogen-binding agent for chronic management of adult and pediatric patients ≥2 years of age with urea cycle disorders (UCDs) who cannot be managed by dietary protein restriction and/or amino acid supplementation alone. RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (e.g., essential amino acids, arginine, citrulline, protein-free calorie supplements).
Limitations of Use:
- RAVICTI is not indicated for the treatment of acute hyperammonemia in patients with UCDs because more rapidly acting interventions are essential to reduce plasma ammonia levels.
- The safety and efficacy of RAVICTI for the treatment of N-acetylglutamate synthase (NAGS) deficiency has not been established.
- The use of RAVICTI in patients <2 months of age is contraindicated.
DOSAGE
Important Instructions
- The recommended dosages for patients switching from sodium phenylbutyrate to RAVICTI and patients naïve to phenylbutyric acid are different . For both subpopulations:
- Give RAVICTI in 3 equally divided dosages, each rounded up to the nearest 0.5 mL.
The maximum total daily dosage is 17.5 mL (19 g).
- RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (e.g., essential amino acids, arginine, citrulline, protein-free calorie supplements).
Switching From Sodium Phenylbutyrate to RAVICTI
- Patients switching from sodium phenylbutyrate to RAVICTI should receive the dosage of RAVICTI that contains the same amount of phenylbutyric acid. The conversion is as follows:
- Total daily dosage of RAVICTI (mL) = total daily dosage of sodium phenylbutyrate (g) x 0.86
Initial Dosage in Phenylbutyrate-Naïve Patients
- The recommended dosage range, based upon body surface area, in patients naïve to phenylbutyrate (PBA) is 4.5 to 11.2 mL/m2/day (5 to 12.4 g/m2/day). For patients with some residual enzyme activity who are not adequately controlled with protein restriction, the recommended starting dosage is 4.5 mL/m2/day.
- In determining the starting dosage of RAVICTI in treatment-naïve patients, consider the patient’s residual urea synthetic capacity, dietary protein requirements, and diet adherence. Dietary protein is approximately 16% nitrogen by weight. Given that approximately 47% of dietary nitrogen is excreted as waste and approximately 70% of an administered PBA dose will be converted to urinary phenylacetylglutamine (U-PAGN), an initial estimated RAVICTI dose for a 24-hour period is 0.6 mL RAVICTI per gram of dietary protein ingested per 24 hour period. The total daily dosage should not exceed 17.5 mL.
Dosage Adjustment and Monitoring
Adjustment based on Plasma Ammonia: Adjust the RAVICTI dosage to produce a fasting plasma ammonia level that is less than half the upper limit of normal (ULN) according to age.
Adjustment Based on Urinary Phenylacetylglutamine: If available, U-PAGN measurements may be used to help guide RAVICTI dose adjustment. Each gram of U-PAGN excreted over 24 hours covers waste nitrogen generated from 1.4 grams of dietary protein. If U-PAGN excretion is insufficient to cover daily dietary protein intake and the fasting ammonia is greater than half the ULN, the RAVICTI dose should be adjusted upward. The amount of dose adjustment should factor in the amount of dietary protein that has not been covered, as indicated by the 24-h U-PAGN level and the estimated RAVICTI dose needed per gram of dietary protein ingested and the maximum total daily dosage i.e., 17.5 mL.
Consider a patient’s use of concomitant medications, such as probenecid, when making dosage adjustment decisions based on U-PAGN. Probenecid may result in a decrease of the urinary excretion of PAGN.
Adjustment Based on Plasma Phenylacetate: If available, measurements of the plasma PAA levels may be useful to guide dosing if symptoms of vomiting, nausea, headache, somnolence, confusion, or sleepiness are present in the absence of high ammonia or intercurrent illness. Ammonia levels must be monitored closely when changing the dose of RAVICTI. The ratio of PAA to PAGN in plasma may provide additional information to assist in dose adjustment decisions. In patients with a high PAA to PAGN ratio, a further increase in RAVICTI dose may not increase PAGN formation, even if plasma PAA concentrations are increased, due to saturation of the conjugation reaction. The PAA to PAGN ratio has been observed to be generally less than 1 in patients without significant PAA accumulation.
Dosage Modifications in Patients with Hepatic Impairment
- For patients with moderate to severe hepatic impairment, the recommended starting dosage is at the lower end of the range
DOSAGE FORMS AND STRENGTHS
- Oral liquid: colorless to pale yellow, 1.1 g/mL of glycerol phenylbutyrate (delivers 1.02 g/mL of phenylbutyrate).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Glycerol phenylbutyrate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Glycerol phenylbutyrate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Glycerol phenylbutyrate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Glycerol phenylbutyrate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Glycerol phenylbutyrate in pediatric patients.
Contraindications
RAVICTI is contraindicated in patients
- Less than 2 months of age. Children <2 months of age may have immature pancreatic exocrine function, which could impair hydrolysis of RAVICTI, leading to impaired absorption of phenylbutyrate and hyperammonemia.
- With known hypersensitivity to phenylbutyrate. Signs of hypersensitivity include wheezing, dyspnea, coughing, hypotension, flushing, nausea, and rash.
Warnings
Neurotoxicity
- The major metabolite of RAVICTI, PAA, is associated with neurotoxicity. Signs and symptoms of PAA neurotoxicity, including somnolence, fatigue, lightheadedness, headache, dysgeusia, hypoacusis, disorientation, impaired memory, and exacerbation of preexisting neuropathy, were observed at plasma PAA concentrations ≥500 µg/mL in a study of cancer patients who were administered IV PAA. In this study, adverse events were reversible.
- In healthy subjects, after administration of 4 mL and 6 mL RAVICTI 3 times daily for 3 days, a dose-dependent increase in all-grade nervous system adverse reactions was observed, even at exposure levels of PAA <100 µg/mL.
- In clinical trials in UCD patients who had been on sodium phenylbutyrate prior to administration of RAVICTI, peak PAA concentrations after dosing with RAVICTI ranged from 1.6 to 178 µg/mL (mean: 39 µg/mL) in adult patients and from 7 to 480 µg/mL (mean: 90 µg/mL) in pediatric patients. Some UCD patients experienced headache, fatigue, symptoms of peripheral neuropathy, seizures, tremor and/or dizziness. No correlation between PAA levels and neurotoxicity symptoms was identified but PAA levels were generally not measured at the time of neurotoxicity symptoms.
- If symptoms of vomiting, nausea, headache, somnolence, confusion, or sleepiness are present in the absence of high ammonia or other intercurrent illnesses, reduce the RAVICTI dosage.
Reduced Phenylbutyrate Absorption in Pancreatic Insufficiency or Intestinal Malabsorption
- Exocrine pancreatic enzymes hydrolyze RAVICTI in the small intestine, separating the active moiety, phenylbutyrate, from glycerol. This process allows phenylbutyrate to be absorbed into the circulation. Low or absent pancreatic enzymes or intestinal disease resulting in fat malabsorption may result in reduced or absent digestion of RAVICTI and/or absorption of phenylbutyrate and reduced control of plasma ammonia. Monitor ammonia levels closely in patients with pancreatic insufficiency or intestinal malabsorption.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- Assessment of adverse reactions was based on exposure of 45 adult patients (31 female and 14 male) with UCD subtype deficiencies of ornithine transcarbamylase (OTC, n=40), carbamyl phosphate synthetase (CPS, n=2), and argininosuccinate synthetase (ASS, n=1) in a randomized, double-blind, active-controlled (RAVICTI vs sodium phenylbutyrate), crossover, 4-week study (Study 1) that enrolled patients ≥18 years of age [see Clinical Studies (14.1)]. One of the 45 patients received only sodium phenylbutyrate prior to withdrawing on day 1 of the study due to an adverse reaction.
- Table 1 summarizes adverse reactions occurring in ≥2 patients treated with RAVICTI or sodium phenylbutyrate. The most common adverse reactions (occurring in ≥10% of patients) reported during short-term treatment with RAVICTI were diarrhea, flatulence, and headache.
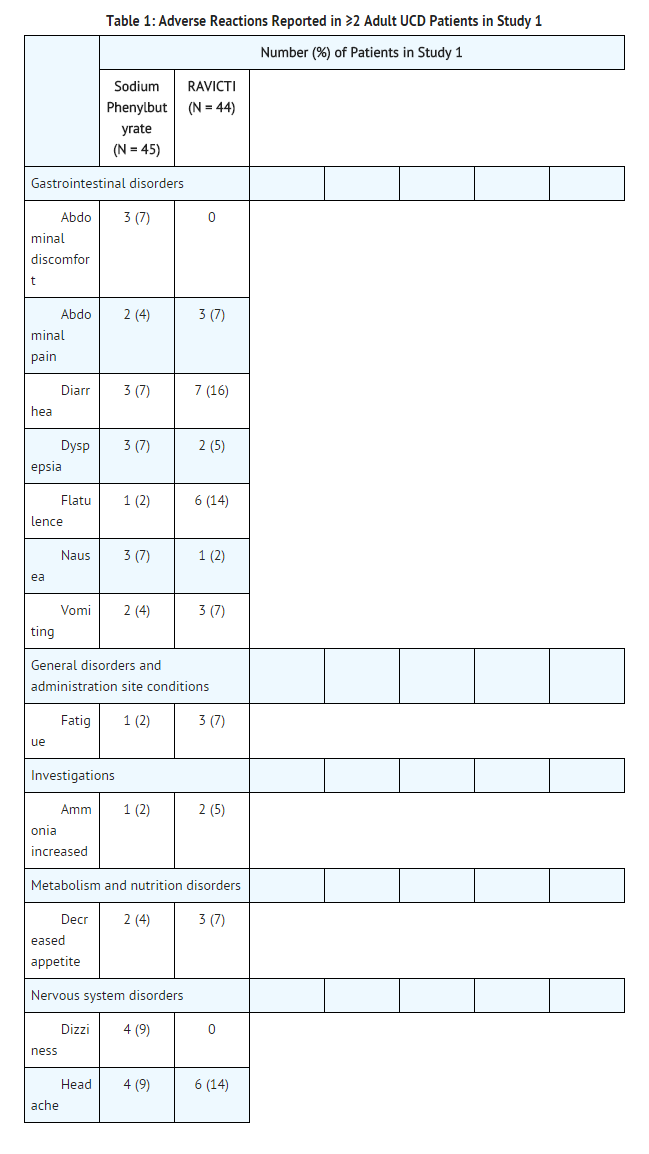
Other Adverse Reactions
- RAVICTI has been evaluated in 77 UCD patients (51 adult and 26 pediatric) in 2 open-label long-term studies, in which 69 patients completed 12 months of treatment with RAVICTI (median exposure = 51 weeks). During these studies there were no deaths.
- Adverse reactions occurring in ≥10% of adult patients were nausea, vomiting, diarrhea, decreased appetite, hyperammonemia, dizziness, headache, and fatigue.
- Adverse reactions occurring in ≥10% of pediatric patients were upper abdominal pain, rash, nausea, vomiting, diarrhea, decreased appetite, hyperammonemia, and headache.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Glycerol phenylbutyrate in the drug label.
Drug Interactions
Potential for Other Drugs to Affect Ammonia
Corticosteroids
- Use of corticosteroids may cause the breakdown of body protein and increase plasma ammonia levels. Monitor ammonia levels closely when corticosteroids and RAVICTI are used concomitantly.
Valproic Acid and Haloperidol
- Hyperammonemia may be induced by haloperidol and by valproic acid. Monitor ammonia levels closely when use of valproic acid or haloperidol is necessary in UCD patients.
Potential for Other Drugs to Affect RAVICTI
Probenecid
- Probenecid may inhibit the renal excretion of metabolites of RAVICTI including PAGN and PAA.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category C
Risk Summary
- There are no adequate and well-controlled studies in pregnant women. In rabbits given glycerol phenylbutyrate at doses up to 2.7 times the dose of 6.87 mL/m2/day in adult patients (based on combined area under the curve [AUCs] for PBA and PAA) during the period of organogenesis, maternal toxicity, but no effects on embryo-fetal development, was observed. In rats given glycerol phenylbutyrate at 1.9 times the dose of 6.87 mL/m2/day in adult patients (based on combined AUCs for PBA and PAA), no adverse embryo-fetal effects were observed. Maternal toxicity, reduced fetal weights, and variations in skeletal development were observed in rats at doses greater than or equal to 5.7 times the dose of 6.87 mL/m2/day in adult patients (based on combined AUCs for PBA and PAA). RAVICTI should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
- Oral administration of glycerol phenylbutyrate during the period of organogenesis up to 350 mg/kg/day in rabbits produced maternal toxicity, but no effects on embryo-fetal development. The dose of 350 mg/kg/day in rabbits is approximately 2.7 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA. In rats, at an oral dose of 300 mg/kg/day of glycerol phenylbutyrate (1.9 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) during the period of organogenesis, no effects on embryo-fetal development were observed. Doses ≥650 mg/kg/day produced maternal toxicity and adverse effects on embryo-fetal development including reduced fetal weights and cervical ribs at the 7th cervical vertebra. The dose of 650 mg/kg/day in rats is approximately 5.7 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA. No developmental abnormalities, effects on growth, or effects on learning and memory were observed in rats through day 92 postpartum following oral administration in pregnant rats with up to 900 mg/kg/day of glycerol phenylbutyrate (8.5 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) during organogenesis and lactation.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Glycerol phenylbutyrate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Glycerol phenylbutyrate during labor and delivery.
Nursing Mothers
- It is not known whether RAVICTI or its metabolites are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions from RAVICTI in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into consideration the importance of the drug to the health of the mother.
Pediatric Use
Patients Between 2 and <18 Years of Age
- The safety and efficacy of RAVICTI in patients 2 to <18 years of age were established in 2 open-label, sodium phenylbutyrate to RAVICTI, fixed-sequence, switchover clinical trials.
Patients ≥2 Months and <2 Years of Age
- The safety and efficacy of RAVICTI in patients 2 months to <2 years of age has not been established. PK and ammonia control were studied in only 4 patients between 2 months and <2 years of age, providing insufficient data to establish a safe and effective dose in this age range.
Patients <2 Months of Age
- RAVICTI is contraindicated in patients <2 months of age. Children <2 months of age may have immature pancreatic exocrine function, which could impair hydrolysis of RAVICTI. Pancreatic lipases may be necessary for intestinal hydrolysis of RAVICTI, allowing release of phenylbutyrate and subsequent formation of PAA, the active moiety. It is not known whether pancreatic and extrapancreatic lipases are sufficient for hydrolysis of RAVICTI. If there is inadequate intestinal hydrolysis of RAVICTI, impaired absorption of phenylbutyrate and hyperammonemia could occur.
Juvenile Animal Study
- In a juvenile rat study with daily oral dosing performed on postpartum day 2 through mating and pregnancy after maturation, terminal body weight was dose-dependently reduced by up to 16% in males and 12% in females. Learning, memory, and motor activity endpoints were not affected. However, fertility (number of pregnant rats) was decreased by up to 25% at ≥650 mg/kg/day (2.6 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA). Embryo toxicity (increased resorptions) occurred at 650 mg/kg/day (2.6 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA) and litter size was reduced at 900 mg/kg/day (3 times the dose of 6.87 mL/m2/day in adult patients, based on combined AUCs for PBA and PAA).
Geriatic Use
- Clinical studies of RAVICTI did not include sufficient numbers of subjects ≥65 years of age to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Glycerol phenylbutyrate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Glycerol phenylbutyrate with respect to specific racial populations.
Renal Impairment
- The efficacy and safety of RAVICTI in patients with renal impairment are unknown. Monitor ammonia levels closely when starting patients with impaired renal function on RAVICTI.
Hepatic Impairment
- No studies were conducted in UCD patients with hepatic impairment. Because conversion of PAA to PAGN occurs in the liver, patients with hepatic impairment may have reduced conversion capability and higher plasma PAA and PAA to PAGN ratio. Therefore, dosage for patients with moderate to severe hepatic impairment should be started at the lower end of the recommended dosing range and should be kept on the lowest dose necessary to control their ammonia levels.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Glycerol phenylbutyrate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Glycerol phenylbutyrate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- RAVICTI should be prescribed by a physician experienced in the management of UCDs. Instruct patients to take RAVICTI with food and to administer directly into the mouth via oral syringe or dosing cup. See the instructions on the use of RAVICTI by nasogastric tube or g-tube .
Preparation for Nasogastric Tube or Gastrostomy Tube Administration
- For patients who have a nasogastric tube or gastrostomy tube in place, administer RAVICTI as follows:
- Utilize an oral syringe to withdraw the prescribed dosage of RAVICTI from the bottle.
- Place the tip of the syringe into to the tip of the gastrostomy/nasogastric tube.
- Utilizing the plunger of the syringe, administer RAVICTI into the tube.
- Flush once with 30 mL of water and allow the flush to drain.
- Flush a second time with an additional 30 mL of water to clear the tube.
Monitoring
There is limited information regarding Monitoring of Glycerol phenylbutyrate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Glycerol phenylbutyrate in the drug label.
Overdosage
- While there is no experience with overdosage in human clinical trials, PAA, a toxic metabolite of RAVICTI, can accumulate in patients who receive an overdose. In case of overdosage, discontinue the drug and contact poison control.
Pharmacology
There is limited information regarding Glycerol phenylbutyrate Pharmacology in the drug label.
Mechanism of Action
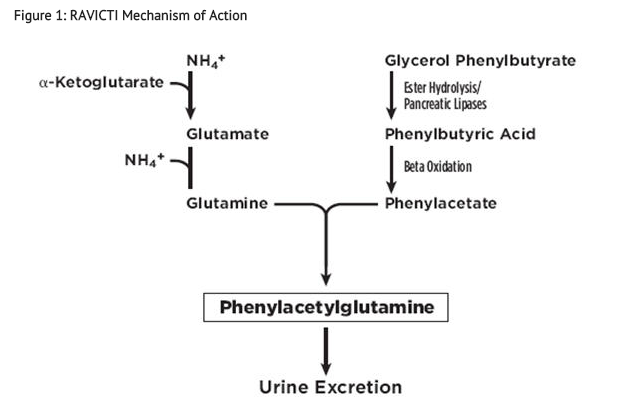
- UCDs are inherited deficiencies of enzymes or transporters necessary for the synthesis of urea from ammonia (NH3, NH4+). Absence of these enzymes or transporters results in the accumulation of toxic levels of ammonia in the blood and brain of affected patients. RAVICTI is a triglyceride containing 3 molecules of phenylbutyrate (PBA). PAA, the major metabolite of PBA, is the active moiety of RAVICTI. PAA conjugates with glutamine (which contains 2 molecules of nitrogen) via acetylation in the liver and kidneys to form PAGN, which is excreted by the kidneys (Figure 1). On a molar basis, PAGN, like urea, contains 2 moles of nitrogen and provides an alternate vehicle for waste nitrogen excretion.
Structure
- RAVICTI (glycerol phenylbutyrate) is a clear, colorless to pale yellow oral liquid. It is insoluble in water and most organic solvents, and it is soluble in dimethylsulfoxide (DMSO) and >65% acetonitrile.
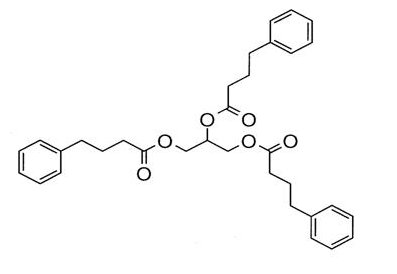
- Glycerol phenylbutyrate is a nitrogen-binding agent. It is a triglyceride containing 3 molecules of PBA linked to a glycerol backbone, the chemical name of which is benzenebutanoic acid, 1', 1' ' –(1,2,3-propanetriyl) ester with a molecular weight of 530.67. It has a molecular formula of C33H38O6. The structural formula is:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Glycerol phenylbutyrate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Glycerol phenylbutyrate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Glycerol phenylbutyrate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Glycerol phenylbutyrate in the drug label.
How Supplied
Storage
There is limited information regarding Glycerol phenylbutyrate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Glycerol phenylbutyrate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Glycerol phenylbutyrate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Glycerol phenylbutyrate in the drug label.
Precautions with Alcohol
- Alcohol-Glycerol phenylbutyrate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Glycerol phenylbutyrate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Glycerol phenylbutyrate |Label Name=Glycerol phenylbutyrate11.png
}}
{{#subobject:
|Label Page=Glycerol phenylbutyrate |Label Name=Glycerol phenylbutyrate11.png
}}