Dipyridamole (injection): Difference between revisions
No edit summary |
No edit summary |
||
| Line 32: | Line 32: | ||
When thallium myocardial perfusion imaging is performed with intravenous dipyridamole, parenteral aminophylline should be readily available for relieving adverse events such as bronchospasm or chest pain. Vital signs should be monitored during, and for 10 to 15 minutes following, the intravenous infusion of dipyridamole and an electrocardiographic tracing should be obtained using at least one chest lead. Should severe chest pain or bronchospasm occur, parenteral aminophylline may be administered by slow intravenous injection (50 to 100 mg over 30 to 60 seconds) in doses ranging from 50 to 250 mg. In the case of severe hypotension, the patient should be placed in a supine position with the head tilted down if necessary, before administration of parenteral aminophylline. If 250 mg of aminophylline does not relieve chest pain symptoms within a few minutes, sublingual nitroglycerin may be administered. If chest pain continues despite use of aminophylline and nitroglycerin, the possibility of myocardial infarction should be considered. If the clinical condition of a patient with an adverse event permits a one minute delay in the administration of parenteral aminophylline, thallium-201 may be injected and allowed to circulate for one minute before the injection of aminophylline. This will allow initial thallium perfusion imaging to be performed before reversal of the pharmacologic effects of dipyridamole on the coronary circulation. | When thallium myocardial perfusion imaging is performed with intravenous dipyridamole, parenteral aminophylline should be readily available for relieving adverse events such as bronchospasm or chest pain. Vital signs should be monitored during, and for 10 to 15 minutes following, the intravenous infusion of dipyridamole and an electrocardiographic tracing should be obtained using at least one chest lead. Should severe chest pain or bronchospasm occur, parenteral aminophylline may be administered by slow intravenous injection (50 to 100 mg over 30 to 60 seconds) in doses ranging from 50 to 250 mg. In the case of severe hypotension, the patient should be placed in a supine position with the head tilted down if necessary, before administration of parenteral aminophylline. If 250 mg of aminophylline does not relieve chest pain symptoms within a few minutes, sublingual nitroglycerin may be administered. If chest pain continues despite use of aminophylline and nitroglycerin, the possibility of myocardial infarction should be considered. If the clinical condition of a patient with an adverse event permits a one minute delay in the administration of parenteral aminophylline, thallium-201 may be injected and allowed to circulate for one minute before the injection of aminophylline. This will allow initial thallium perfusion imaging to be performed before reversal of the pharmacologic effects of dipyridamole on the coronary circulation. | ||
|clinicalTrials=Adverse reaction information concerning intravenous dipyridamole is derived from a study of 3911 patients in which intravenous dipyridamole was used as an adjunct to thallium myocardial perfusion imaging and from spontaneous reports of adverse reactions and the published literature. | |clinicalTrials=Adverse reaction information concerning intravenous dipyridamole is derived from a study of 3911 patients in which intravenous dipyridamole was used as an adjunct to thallium myocardial perfusion imaging and from spontaneous reports of adverse reactions and the published literature. | ||
| Line 40: | Line 39: | ||
Adverse reactions occurring in greater than 1% of the patients in the study are shown in the following table: | Adverse reactions occurring in greater than 1% of the patients in the study are shown in the following table: | ||
[[File:Dipyridamole_injection1.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Less common adverse reactions occurring in 1% or less of the patients within the study included: | |||
Cardiovascular System: Electrocardiographic abnormalities unspecified (0.8%), arrhythmia unspecified (0.6%), palpitation (0.3%), ventricular tachycardia (0.2% see WARNINGS), bradycardia (0.2%), myocardial infarction (0.1% see WARNINGS), AV block (0.1%), syncope (0.1%), orthostatic hypotension (0.1%), atrial fibrillation (0.1%), supraventricular tachycardia (0.1%), ventricular arrhythmia unspecified (0.03% see WARNINGS), heart block unspecified (0.03%), cardiomyopathy (0.03%), edema (0.03%). | |||
Central and Peripheral Nervous System: Hypothesia (0.5%), hypertonia (0.3%), nervousness/anxiety (0.2%), tremor (0.1%), abnormal coordination (0.03%), somnolence (0.03%), dysphonia (0.03%), migraine (0.03%), vertigo (0.03%). | |||
Gastrointestinal System: Dyspepsia (1%), dry mouth (0.8%), abdominal pain (0.7%), flatulence (0.6%), vomiting (0.4%), eructation (0.1%), dysphagia (0.03%), tenesmus (0.03%), appetite increased (0.03%). | |||
Respiratory System: Pharyngitis (0.3%), bronchospasm (0.2% see WARNINGS), hyperventilation (0.1%), rhinitis (0.1%), coughing (0.03%), pleural pain (0.03%). | |||
Other: Myalgia (0.9%), back pain (0.6%), injection site reaction unspecified (0.4%), diaphoresis (0.4%), asthenia (0.3%), malaise (0.3%), arthralgia (0.3%), injection site pain (0.1%), rigor (0.1%), earache (0.1%), tinnitus (0.1%), vision abnormalities unspecified (0.1%), dysgeusia (0.1%), thirst (0.03%), depersonalization (0.03%), eye pain (0.03%), renal pain (0.03%), perineal pain (0.03%), breast pain (0.03%), intermittent claudication (0.03%), leg cramping (0.03%). In additional postmarketing experience, there have been rare reports of allergic reaction including urticaria, pruritus, dermatitis and rash. | |||
|drugInteractions=Oral maintenance theophylline and other xanthine derivatives such as caffeine may abolish the coronary vasodilatation induced by intravenous dipyridamole administration. This could lead to a false negative thallium imaging result (see CLINICAL PHARMACOLOGY, Mechanism of Action). | |drugInteractions=Oral maintenance theophylline and other xanthine derivatives such as caffeine may abolish the coronary vasodilatation induced by intravenous dipyridamole administration. This could lead to a false negative thallium imaging result (see CLINICAL PHARMACOLOGY, Mechanism of Action). | ||
| Line 49: | Line 59: | ||
*Calculation based on assumed body weight of 50 kg. | *Calculation based on assumed body weight of 50 kg. | ||
|useInNursing=Dipyridamole is excreted in human milk. | |useInNursing=Dipyridamole is excreted in human milk. | ||
|overdose=No cases of overdosage in humans have been reported. It is unlikely that overdosage will occur because of the nature of use (i.e., single intravenous administration in controlled settings). See WARNINGS. | |||
|mechAction=Dipyridamole is a coronary vasodilator in man. The mechanism of vasodilation has not been fully elucidated, but may result from inhibition of uptake of adenosine, an important mediator of coronary vasodilation. The vasodilatory effects of dipyridamole are abolished by administration of the adenosine receptor antagonist theophylline. | |||
How dipyridamole-induced vasodilation leads to abnormalities in thallium distribution and ventricular function is also uncertain but presumably represents a “steal” phenomenon in which relatively intact vessels dilate, and sustain enhanced flow, leaving reduced pressure and flow across areas of hemodynamically important coronary vascular constriction. | |||
|structure=Dipyridamole is a coronary vasodilator described as 2,6 bis-(diethanolamino)-4,8-dipiperidino-pyrimido-(5,4-d) pyrimidine. It has the molecular formula C24H40N8O4 and the following structural formula: | |||
[[File:Dipyridamole_injection2.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
M. W. 504.64 | |||
Dipyridamole injection is an odorless, pale yellow liquid which can be diluted in sodium chloride injection or dextrose injection for intravenous administration. | |||
Each mL of sterile solution for intravenous administration contains 5 mg dipyridamole, with 2 mg tartaric acid, and 50 mg polyethylene glycol 600. pH is adjusted to 2.2 to 3.2 with hydrochloric acid. | |||
|PK=Plasma dipyridamole concentrations decline in a triexponential fashion following intravenous infusion of dipyridamole, with half-lives averaging 3 to 12 minutes, 33 to 62 minutes, and 11.6 to 15 hours. Two minutes following a 0.568 mg/kg dose of intravenous dipyridamole administered as a 4-minute infusion, the mean dipyridamole serum concentration is 4.6±1.3 mcg/mL. The average plasma protein binding of dipyridamole is approximately 99%, primarily to α1 -glycoprotein. Dipyridamole is metabolized in the liver to the glucuronic acid conjugate and excreted with the bile. The average total body clearance is 2.3 to 3.5 mL/min/kg, with an apparent volume of distribution at steady state of 1 to 2.5 L/kg and a central apparent volume of 3 to 5 liters. | |||
|nonClinToxic=Carcinogenesis, Mutagenesis, Impairment of Fertility: | |nonClinToxic=Carcinogenesis, Mutagenesis, Impairment of Fertility: | ||
In studies in which dipyridamole was administered in the feed at doses of up to 75 mg/kg/day (9.4 times* the maximum recommended daily human oral dose) in mice (up to 128 weeks in males and up to 142 weeks in females) and rats (up to 111 weeks in males and females), there was no evidence of drug related carcinogenesis. Mutagenicity tests of dipyridamole with bacterial and mammalian cell systems were negative. There was no evidence of impaired fertility when dipyridamole was administered to male and female rats at oral doses up to 500 mg/kg/day (63 times* the maximum recommended daily human oral dose). A significant reduction in number of corpora lutea with consequent reduction in implantations and live fetuses was, however, observed at 1250 mg/kg/day. | In studies in which dipyridamole was administered in the feed at doses of up to 75 mg/kg/day (9.4 times* the maximum recommended daily human oral dose) in mice (up to 128 weeks in males and up to 142 weeks in females) and rats (up to 111 weeks in males and females), there was no evidence of drug related carcinogenesis. Mutagenicity tests of dipyridamole with bacterial and mammalian cell systems were negative. There was no evidence of impaired fertility when dipyridamole was administered to male and female rats at oral doses up to 500 mg/kg/day (63 times* the maximum recommended daily human oral dose). A significant reduction in number of corpora lutea with consequent reduction in implantations and live fetuses was, however, observed at 1250 mg/kg/day. | ||
*Calculation based on assumed body weight of 50 kg. | *Calculation based on assumed body weight of 50 kg. | ||
|howSupplied=Dipyridamole Injection, is available in 2 mL and 10 mL ampules: | |||
10 mg/2 mL (5 mg per mL) Box of 10 (List 2043). | |||
50 mg/10 mL (5 mg per mL) Box of 10 (List 2043). | |||
|storage=Store between 15°C (59°F) − 25°C (77°F). | |||
|alcohol=Alcohol-Dipyridamole (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Dipyridamole (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 17:07, 4 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dipyridamole (injection) is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Dipyridamole injection is indicated as an alternative to exercise in thallium myocardial perfusion imaging for the evaluation of coronary artery disease in patients who cannot exercise adequately.
In a study of about 1100 patients who underwent coronary arteriography and dipyridamole injection assisted thallium imaging, the results of both tests were interpreted blindly and the sensitivity and specificity of the dipyridamole thallium study in predicting the angiographic outcome were calculated. The sensitivity of the dipyridamole test (true positive dipyridamole divided by the total number of patients with positive angiography) was about 85%. The specificity (true negative divided by the number of patients with negative angiograms) was about 50%.
In a subset of patients who had exercise thallium imaging as well as dipyridamole thallium imaging, sensitivity and specificity of the two tests was almost identical.
Dosage
The dose of intravenous dipyridamole as an adjunct to thallium myocardial perfusion imaging should be adjusted according to the weight of the patient. The recommended dose is 0.142 mg/kg/minute (0.57 mg/kg total) infused over 4 minutes. Although the maximum tolerated dose has not been determined, clinical experience suggests that a total dose beyond 60 mg is not needed for any patient.
Prior to intravenous administration, dipyridamole injection should be diluted in at least a 1:2 ratio with sodium chloride injection, 0.45%; sodium chloride injection, 0.9%; or dextrose injection, 5% for a total volume of approximately 20 to 50 mL. Infusion of undiluted dipyridamole may cause local irritation.
Thallium-201 should be injected within 5 minutes following the 4-minute infusion of dipyridamole.
Do not mix dipyridamole injection with other drugs in the same syringe or infusion container.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dipyridamole (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dipyridamole (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dipyridamole (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dipyridamole (injection) in pediatric patients.
Contraindications
Hypersensitivity to dipyridamole.
Warnings
Serious adverse reactions associated with the administration of intravenous dipyridamole have included cardiac death, fatal and non-fatal myocardial infarction, ventricular fibrillation, symptomatic ventricular tachycardia, stroke, transient cerebral ischemia, seizures, anaphylactoid reaction and bronchospasm. There have been reported cases of asystole, sinus node arrest, sinus node depression and conduction block. Patients with abnormalities of cardiac impulse formation/conduction or severe coronary artery disease may be at increased risk for these events.
In a study of 3911 patients given intravenous dipyridamole as an adjunct to thallium myocardial perfusion imaging, two types of serious adverse events were reported: 1) four cases of myocardial infarction (0.1%), two fatal (0.05%); and two non-fatal (0.05%); and 2) six cases of severe bronchospasm (0.2%). Although the incidence of these serious adverse events was small (0.3%, 10 of 3911), the potential clinical information to be gained through use of intravenous dipyridamole thallium imaging (see INDICATIONS AND USAGE noting the rate of false positive and false negative results) must be weighed against the risk to the patient. Patients with a history of unstable angina may be at a greater risk for severe myocardial ischemia. Patients with a history of asthma may be at a greater risk for bronchospasm during dipyridamole use.
When thallium myocardial perfusion imaging is performed with intravenous dipyridamole, parenteral aminophylline should be readily available for relieving adverse events such as bronchospasm or chest pain. Vital signs should be monitored during, and for 10 to 15 minutes following, the intravenous infusion of dipyridamole and an electrocardiographic tracing should be obtained using at least one chest lead. Should severe chest pain or bronchospasm occur, parenteral aminophylline may be administered by slow intravenous injection (50 to 100 mg over 30 to 60 seconds) in doses ranging from 50 to 250 mg. In the case of severe hypotension, the patient should be placed in a supine position with the head tilted down if necessary, before administration of parenteral aminophylline. If 250 mg of aminophylline does not relieve chest pain symptoms within a few minutes, sublingual nitroglycerin may be administered. If chest pain continues despite use of aminophylline and nitroglycerin, the possibility of myocardial infarction should be considered. If the clinical condition of a patient with an adverse event permits a one minute delay in the administration of parenteral aminophylline, thallium-201 may be injected and allowed to circulate for one minute before the injection of aminophylline. This will allow initial thallium perfusion imaging to be performed before reversal of the pharmacologic effects of dipyridamole on the coronary circulation.
Adverse Reactions
Clinical Trials Experience
Adverse reaction information concerning intravenous dipyridamole is derived from a study of 3911 patients in which intravenous dipyridamole was used as an adjunct to thallium myocardial perfusion imaging and from spontaneous reports of adverse reactions and the published literature.
Serious adverse events (cardiac death, fatal and non-fatal myocardial infarction, ventricular fibrillation, asystole, sinus node arrest, symptomatic ventricular tachycardia, stroke, transient cerebral ischemia, seizures, anaphylactoid reaction and bronchospasm) are described above (see WARNINGS).
In the study of 3911 patients, the most frequent adverse reactions were: chest pain/angina pectoris (19.7%), electrocardiographic changes (most commonly ST-T changes) (15.9%), headache (12.2%), and dizziness (11.8%).
Adverse reactions occurring in greater than 1% of the patients in the study are shown in the following table:

Less common adverse reactions occurring in 1% or less of the patients within the study included:
Cardiovascular System: Electrocardiographic abnormalities unspecified (0.8%), arrhythmia unspecified (0.6%), palpitation (0.3%), ventricular tachycardia (0.2% see WARNINGS), bradycardia (0.2%), myocardial infarction (0.1% see WARNINGS), AV block (0.1%), syncope (0.1%), orthostatic hypotension (0.1%), atrial fibrillation (0.1%), supraventricular tachycardia (0.1%), ventricular arrhythmia unspecified (0.03% see WARNINGS), heart block unspecified (0.03%), cardiomyopathy (0.03%), edema (0.03%).
Central and Peripheral Nervous System: Hypothesia (0.5%), hypertonia (0.3%), nervousness/anxiety (0.2%), tremor (0.1%), abnormal coordination (0.03%), somnolence (0.03%), dysphonia (0.03%), migraine (0.03%), vertigo (0.03%).
Gastrointestinal System: Dyspepsia (1%), dry mouth (0.8%), abdominal pain (0.7%), flatulence (0.6%), vomiting (0.4%), eructation (0.1%), dysphagia (0.03%), tenesmus (0.03%), appetite increased (0.03%).
Respiratory System: Pharyngitis (0.3%), bronchospasm (0.2% see WARNINGS), hyperventilation (0.1%), rhinitis (0.1%), coughing (0.03%), pleural pain (0.03%).
Other: Myalgia (0.9%), back pain (0.6%), injection site reaction unspecified (0.4%), diaphoresis (0.4%), asthenia (0.3%), malaise (0.3%), arthralgia (0.3%), injection site pain (0.1%), rigor (0.1%), earache (0.1%), tinnitus (0.1%), vision abnormalities unspecified (0.1%), dysgeusia (0.1%), thirst (0.03%), depersonalization (0.03%), eye pain (0.03%), renal pain (0.03%), perineal pain (0.03%), breast pain (0.03%), intermittent claudication (0.03%), leg cramping (0.03%). In additional postmarketing experience, there have been rare reports of allergic reaction including urticaria, pruritus, dermatitis and rash.
Postmarketing Experience
There is limited information regarding Dipyridamole (injection) Postmarketing Experience in the drug label.
Drug Interactions
Oral maintenance theophylline and other xanthine derivatives such as caffeine may abolish the coronary vasodilatation induced by intravenous dipyridamole administration. This could lead to a false negative thallium imaging result (see CLINICAL PHARMACOLOGY, Mechanism of Action).
Myasthenia gravis patients receiving therapy with cholinesterase inhibitors may experience worsening of their disease in the presence of dipyridamole.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B Reproduction studies performed in mice and rats at daily oral doses of up to 125 mg/kg (15.6 times* the maximum recommended daily human oral dose) and in rabbits at daily oral doses of up to 20 mg/kg (2.5 times* the maximum recommended daily human oral dose) have revealed no evidence of impaired embryonic development due to dipyridamole. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human responses, this drug should be used during pregnancy only if clearly needed.
- Calculation based on assumed body weight of 50 kg.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dipyridamole (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dipyridamole (injection) during labor and delivery.
Nursing Mothers
Dipyridamole is excreted in human milk.
Pediatric Use
There is no FDA guidance on the use of Dipyridamole (injection) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Dipyridamole (injection) in geriatric settings.
Gender
There is no FDA guidance on the use of Dipyridamole (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dipyridamole (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dipyridamole (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dipyridamole (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dipyridamole (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dipyridamole (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Dipyridamole (injection) Administration in the drug label.
Monitoring
There is limited information regarding Dipyridamole (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Dipyridamole (injection) and IV administrations.
Overdosage
No cases of overdosage in humans have been reported. It is unlikely that overdosage will occur because of the nature of use (i.e., single intravenous administration in controlled settings). See WARNINGS.
Pharmacology
There is limited information regarding Dipyridamole (injection) Pharmacology in the drug label.
Mechanism of Action
Dipyridamole is a coronary vasodilator in man. The mechanism of vasodilation has not been fully elucidated, but may result from inhibition of uptake of adenosine, an important mediator of coronary vasodilation. The vasodilatory effects of dipyridamole are abolished by administration of the adenosine receptor antagonist theophylline.
How dipyridamole-induced vasodilation leads to abnormalities in thallium distribution and ventricular function is also uncertain but presumably represents a “steal” phenomenon in which relatively intact vessels dilate, and sustain enhanced flow, leaving reduced pressure and flow across areas of hemodynamically important coronary vascular constriction.
Structure
Dipyridamole is a coronary vasodilator described as 2,6 bis-(diethanolamino)-4,8-dipiperidino-pyrimido-(5,4-d) pyrimidine. It has the molecular formula C24H40N8O4 and the following structural formula:
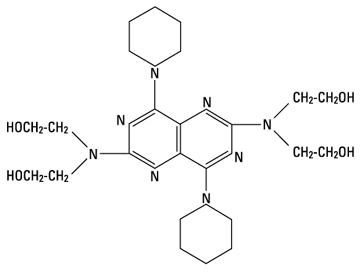
M. W. 504.64
Dipyridamole injection is an odorless, pale yellow liquid which can be diluted in sodium chloride injection or dextrose injection for intravenous administration.
Each mL of sterile solution for intravenous administration contains 5 mg dipyridamole, with 2 mg tartaric acid, and 50 mg polyethylene glycol 600. pH is adjusted to 2.2 to 3.2 with hydrochloric acid.
Pharmacodynamics
There is limited information regarding Dipyridamole (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
Plasma dipyridamole concentrations decline in a triexponential fashion following intravenous infusion of dipyridamole, with half-lives averaging 3 to 12 minutes, 33 to 62 minutes, and 11.6 to 15 hours. Two minutes following a 0.568 mg/kg dose of intravenous dipyridamole administered as a 4-minute infusion, the mean dipyridamole serum concentration is 4.6±1.3 mcg/mL. The average plasma protein binding of dipyridamole is approximately 99%, primarily to α1 -glycoprotein. Dipyridamole is metabolized in the liver to the glucuronic acid conjugate and excreted with the bile. The average total body clearance is 2.3 to 3.5 mL/min/kg, with an apparent volume of distribution at steady state of 1 to 2.5 L/kg and a central apparent volume of 3 to 5 liters.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility: In studies in which dipyridamole was administered in the feed at doses of up to 75 mg/kg/day (9.4 times* the maximum recommended daily human oral dose) in mice (up to 128 weeks in males and up to 142 weeks in females) and rats (up to 111 weeks in males and females), there was no evidence of drug related carcinogenesis. Mutagenicity tests of dipyridamole with bacterial and mammalian cell systems were negative. There was no evidence of impaired fertility when dipyridamole was administered to male and female rats at oral doses up to 500 mg/kg/day (63 times* the maximum recommended daily human oral dose). A significant reduction in number of corpora lutea with consequent reduction in implantations and live fetuses was, however, observed at 1250 mg/kg/day.
- Calculation based on assumed body weight of 50 kg.
Clinical Studies
There is limited information regarding Dipyridamole (injection) Clinical Studies in the drug label.
How Supplied
Dipyridamole Injection, is available in 2 mL and 10 mL ampules:
10 mg/2 mL (5 mg per mL) Box of 10 (List 2043).
50 mg/10 mL (5 mg per mL) Box of 10 (List 2043).
Storage
Store between 15°C (59°F) − 25°C (77°F).
Images
Drug Images
{{#ask: Page Name::Dipyridamole (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dipyridamole (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Dipyridamole (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Dipyridamole (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Dipyridamole (injection) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Dipyridamole (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.