Diltiazem (oral): Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=Calcium Channel Blocker | |drugClass=Calcium Channel Blocker | ||
|indication=[[chronic stable angina]] and [[angina]] due to [[coronary artery spasm]] (tablet or capsule), [[hypertension]] (capsule only), [[Atrial Fibrillation]] or [[Atrial Flutter]], [[Paroxysmal Supraventricular Tachycardia]] (injection only) | |indication=[[chronic stable angina]] and [[angina]] due to [[coronary artery spasm]] (tablet or capsule), [[hypertension]] (capsule only), [[Atrial Fibrillation]] or [[Atrial Flutter]], [[Paroxysmal Supraventricular Tachycardia]] (injection only) | ||
|adverseReactions=[[Bradyarrhythmia]], Peripheral [[edema]], [[Dizziness]], [[Headache]], [[Cough]], [[Fatigue]] | |adverseReactions=[[Bradyarrhythmia]], Peripheral [[edema]], [[Dizziness]], [[Headache]], [[Cough]], [[Fatigue]] | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=<h4>[[chronic stable angina]]</ | |fdaLIADAdult=<h4>[[chronic stable angina]]</h4> | ||
* Indication ('''tablet and capsule''') | * Indication ('''tablet and capsule''') | ||
| Line 41: | Line 41: | ||
:* Temporary control of rapid ventricular rate in atrial fibrillation or atrial flutter. It should not be used in patients with atrial fibrillation or atrial flutter associated with an accessory bypass tract such as in Wolff-Parkinson-White (WPW) syndrome or short PR syndrome. | :* Temporary control of rapid ventricular rate in atrial fibrillation or atrial flutter. It should not be used in patients with atrial fibrillation or atrial flutter associated with an accessory bypass tract such as in Wolff-Parkinson-White (WPW) syndrome or short PR syndrome. | ||
<H4>Concomitant use with Other Cardiovascular Agents.</H4> | |||
* 1.Sublingual [[Nitroglycerin]] (NTG). May be taken as required to abort acute anginal attacks during diltiazem hydrochloride therapy. | |||
* 2. Prophylactic Nitrate Therapy. Diltiazem hydrochloride may be safely co-administered with short- and long-acting nitrates. | |||
* 3.[[Beta-blockers]] (See WARNINGS and PRECAUTIONS.) | |||
* 4.Antihypertensives. Diltiazem hydrochloride has an additive antihypertensive effect when used with other antihypertensive agents. Therefore, the dosage of diltiazem hydrochloride or the concomitant antihypertensives may need to be adjusted when adding one to the other. | |||
* [[Hypertensive]] or anginal patients who are treated with other formulations of diltiazem can safely be switched to diltiazem hydrochloride extended-release capsules at the nearest equivalent total daily dose. Subsequent titration to higher or lower doses may, however, be necessary and should be initiated as clinically indicated. | |||
<h4>[[Paroxysmal Supraventricular Tachycardia]]</h4> | <h4>[[Paroxysmal Supraventricular Tachycardia]]</h4> | ||
| Line 56: | Line 65: | ||
:* Symptoms associated with the arrhythmia were improved in conjunction with decreased heart rate or conversion to normal sinus rhythm following administration of diltiazem hydrochloride injection. | :* Symptoms associated with the arrhythmia were improved in conjunction with decreased heart rate or conversion to normal sinus rhythm following administration of diltiazem hydrochloride injection. | ||
|offLabelAdultGuideSupport= | * Dosing information | ||
:* Direct Intravenous Single Injections (Bolus) | |||
::* The initial dose of diltiazem hydrochloride injection should be 0.25 mg/kg actual body weight as a bolus administered over 2 minutes (20 mg is a reasonable dose for the average patient). If response is inadequate, a second dose may be administered after 15 minutes. The second bolus dose of diltiazem hydrochloride injection should be 0.35 mg/kg actual body weight administered over 2 minutes (25 mg is a reasonable dose for the average patient). Subsequent intravenous bolus doses should be individualized for each patient. Patients with low body weights should be dosed on a mg/kg basis. Some patients may respond to an initial dose of 0.15 mg/kg, although duration of action may be shorter. Experience with this dose is limited. | |||
:* Continuous Intravenous Infusion | |||
::* For continued reduction of the heart rate (up to 24 hours) in patients with atrial fibrillation or atrial flutter, an intravenous infusion of diltiazem hydrochloride may be administered. Immediately following bolus administration of 20 mg (0.25 mg/kg) or 25 mg (0.35 mg/kg) diltiazem hydrochloride injection and reduction of heart rate, begin an intravenous infusion of diltiazem hydrochloride. The recommended initial infusion rate of diltiazem hydrochloride is 10 mg/h. Some patients may maintain response to an initial rate of 5 mg/h. The infusion rate may be increased in 5 mg/h increments up to 15 mg/h as needed, if further reduction in heart rate is required. The infusion may be maintained for up to 24 hours. | |||
::* Diltiazem shows dose-dependent, non-linear pharmacokinetics. Duration of infusion longer than 24 hours and infusion rates greater than 15 mg/h have not been studied. Therefore, infusion duration exceeding 24 hours and infusion rates exceeding 15 mg/h are not recommended. | |||
::* '''Dilution''': To prepare diltiazem hydrochloride injection for continuous intravenous infusion, aseptically transfer the appropriate quantity (see chart) of diltiazem hydrochloride injection to the desired volume of either Normal Saline, D5W, or D5W/0.45% NaCl. Mix thoroughly. Use within 24 hours. Keep refrigerated until use. | |||
[[File:Diltiazem_administration_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|offLabelAdultGuideSupport=<H4>Unstable Angina</H4> | |||
* Developed by: [[American College of Cardiology|American College of Cardiology (ACC)]] and [[American Heart Association|American Heart Association (AHA)]] | |||
* Class of Recommendation: [[ACC AHA guidelines classification scheme#Class I: Benefit >>> Risk|Class I]] | |||
* Level of Evidence: Not applicable | |||
* Dosing Information | |||
:* '''20 mg IV ''' | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Diltiazem sandbox in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Diltiazem sandbox in adult patients. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Diltiazem sandbox in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Diltiazem sandbox in pediatric patients. | ||
Revision as of 18:25, 1 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Diltiazem (oral) is a Calcium Channel Blocker that is FDA approved for the {{{indicationType}}} of chronic stable angina and angina due to coronary artery spasm (tablet or capsule), hypertension (capsule only), Atrial Fibrillation or Atrial Flutter, Paroxysmal Supraventricular Tachycardia (injection only). Common adverse reactions include Bradyarrhythmia, Peripheral edema, Dizziness, Headache, Cough, Fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
chronic stable angina
- Indication (tablet and capsule)
- Exertional Angina Pectoris Due to Atherosclerotic Coronary Artery Disease or Angina Pectoris at Rest Due to Coronary Artery Spasm
- Dosing information (tablet)
- Dosage must be adjusted to each patient's needs.
- Initial dosage: 30 mg PO qid, before meals and at bedtime,
- Dosage should be increased gradually (given in divided doses three or four times daily) at 1- to 2-day intervals until optimum response is obtained.
- Average optimum dosage range: 180 to 360 mg/day. There are no available data concerning dosage requirements in patients with impaired renal or hepatic function. If the drug must be used in such patients, titration should be carried out with particular caution.
- Dosing information (capsule)
- Dosages for the treatment of angina should be adjusted to each patient's needs.
- Initial dosage: 120 mg to 180 mg PO qd.
- Individual patients may respond to higher doses of up to 540 mg once daily. When necessary, titration should be carried out over 7 to 14 days.
Hypertension
- Dosing information (capsule only)
- Dosage needs to be adjusted by titration to individual patient needs.
- When used as monotherapy, usual starting doses : 120 to 240 mg PO qd.
- Maximum antihypertensive effect is usually observed by 14 days of chronic therapy; therefore, dosage adjustments should be scheduled accordingly.
- Usual dosage range : 120 to 540 mg PO qd. Current clinical experience with ‘’‘540 mg’‘’ dose is limited; however, the dose may be increased to ‘’‘540 mg once daily’‘’.
Atrial Fibrillation or Atrial Flutter
- Indication (injection only)
- Temporary control of rapid ventricular rate in atrial fibrillation or atrial flutter. It should not be used in patients with atrial fibrillation or atrial flutter associated with an accessory bypass tract such as in Wolff-Parkinson-White (WPW) syndrome or short PR syndrome.
Concomitant use with Other Cardiovascular Agents.
- 1.Sublingual Nitroglycerin (NTG). May be taken as required to abort acute anginal attacks during diltiazem hydrochloride therapy.
- 2. Prophylactic Nitrate Therapy. Diltiazem hydrochloride may be safely co-administered with short- and long-acting nitrates.
- 3.Beta-blockers (See WARNINGS and PRECAUTIONS.)
- 4.Antihypertensives. Diltiazem hydrochloride has an additive antihypertensive effect when used with other antihypertensive agents. Therefore, the dosage of diltiazem hydrochloride or the concomitant antihypertensives may need to be adjusted when adding one to the other.
- Hypertensive or anginal patients who are treated with other formulations of diltiazem can safely be switched to diltiazem hydrochloride extended-release capsules at the nearest equivalent total daily dose. Subsequent titration to higher or lower doses may, however, be necessary and should be initiated as clinically indicated.
Paroxysmal Supraventricular Tachycardia
- Indication (injection only)
- Rapid conversion of paroxysmal supra ventricular tachycardias (PSVT) to sinus rhythm. This includes AV nodal reentrant tachycardias and reciprocating tachycardias associated with an extranodal accessory pathway such as the WPW syndrome or short PR syndrome. Unless otherwise contraindicated, appropriate vagal maneuvers should be attempted prior to administration of diltiazem hydrochloride injection.
- The use of diltiazem hydrochloride injection should be undertaken with caution when the patient is compromised hemodynamically or is taking other drugs that decrease any or all of the following: peripheral resistance, myocardial filling, myocardial contractility, or electrical impulse propagation in the myocardium.
- For either indication and particularly when employing continuous intravenous infusion, the setting should include continuous monitoring of the ECG and frequent measurement of blood pressure. A defibrillator and emergency equipment should be readily available.
In domestic controlled trials in patients with atrial fibrillation or atrial flutter, bolus administration of diltiazem hydrochloride injection was effective in reducing heart rate by at least 20% in 95% of patients. Diltiazem hydrochloride injection rarely converts atrial fibrillation or atrial flutter to normal sinus rhythm. :* :* :* :* :* Following administration of one or two intravenous bolus doses of diltiazem injection, response usually occurs within 3 minutes and maximal heart rate reduction generally occurs in 2 to 7 minutes. Heart rate reduction may last from 1 to 3 hours. If hypotension occurs, it is generally short-lived, but may last from 1 to 3 hours.
- A 24-hour continuous infusion of diltiazem injection in the treatment of atrial fibrillation or atrial flutter maintained at least a 20% heart rate reduction during the infusion in 83% of patients.
- Upon discontinuation of infusion, heart rate reduction may last from 0.5 hours to more than 10 hours (median duration 7 hours). Hypotension, if it occurs, may be similarly persistent.
- In the controlled clinical trials, 3.2% of patients required some form of intervention (typically, use of intravenous fluids or the Trendelenburg position) for blood pressure support following diltiazem hydrochloride injection.
- In domestic controlled trials, bolus administration of diltiazem hydrochloride injection was effective in converting PSVT to normal sinus rhythm in 88% of patients within 3 minutes of the first or second bolus dose.
- Symptoms associated with the arrhythmia were improved in conjunction with decreased heart rate or conversion to normal sinus rhythm following administration of diltiazem hydrochloride injection.
- Dosing information
- Direct Intravenous Single Injections (Bolus)
- The initial dose of diltiazem hydrochloride injection should be 0.25 mg/kg actual body weight as a bolus administered over 2 minutes (20 mg is a reasonable dose for the average patient). If response is inadequate, a second dose may be administered after 15 minutes. The second bolus dose of diltiazem hydrochloride injection should be 0.35 mg/kg actual body weight administered over 2 minutes (25 mg is a reasonable dose for the average patient). Subsequent intravenous bolus doses should be individualized for each patient. Patients with low body weights should be dosed on a mg/kg basis. Some patients may respond to an initial dose of 0.15 mg/kg, although duration of action may be shorter. Experience with this dose is limited.
- Continuous Intravenous Infusion
- For continued reduction of the heart rate (up to 24 hours) in patients with atrial fibrillation or atrial flutter, an intravenous infusion of diltiazem hydrochloride may be administered. Immediately following bolus administration of 20 mg (0.25 mg/kg) or 25 mg (0.35 mg/kg) diltiazem hydrochloride injection and reduction of heart rate, begin an intravenous infusion of diltiazem hydrochloride. The recommended initial infusion rate of diltiazem hydrochloride is 10 mg/h. Some patients may maintain response to an initial rate of 5 mg/h. The infusion rate may be increased in 5 mg/h increments up to 15 mg/h as needed, if further reduction in heart rate is required. The infusion may be maintained for up to 24 hours.
- Diltiazem shows dose-dependent, non-linear pharmacokinetics. Duration of infusion longer than 24 hours and infusion rates greater than 15 mg/h have not been studied. Therefore, infusion duration exceeding 24 hours and infusion rates exceeding 15 mg/h are not recommended.
- Dilution: To prepare diltiazem hydrochloride injection for continuous intravenous infusion, aseptically transfer the appropriate quantity (see chart) of diltiazem hydrochloride injection to the desired volume of either Normal Saline, D5W, or D5W/0.45% NaCl. Mix thoroughly. Use within 24 hours. Keep refrigerated until use.
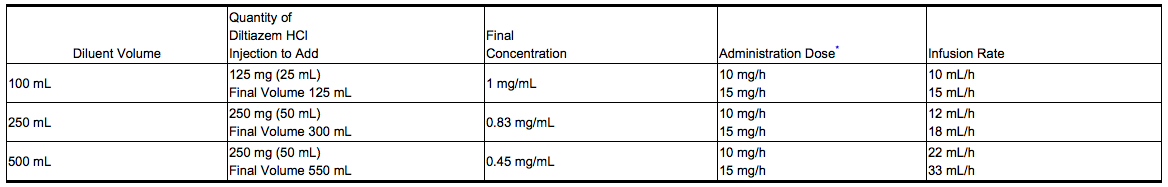
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Unstable Angina
- Developed by: American College of Cardiology (ACC) and American Heart Association (AHA)
- Class of Recommendation: Class I
- Level of Evidence: Not applicable
- Dosing Information
- 20 mg IV
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diltiazem sandbox in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Diltiazem (oral) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Diltiazem sandbox in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diltiazem sandbox in pediatric patients.
Contraindications
There is limited information regarding Diltiazem (oral) Contraindications in the drug label.
Warnings
There is limited information regarding Diltiazem (oral) Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Diltiazem (oral) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Diltiazem (oral) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Diltiazem (oral) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Diltiazem (oral) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Diltiazem (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Diltiazem (oral) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Diltiazem (oral) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Diltiazem (oral) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Diltiazem (oral) in geriatric settings.
Gender
There is no FDA guidance on the use of Diltiazem (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Diltiazem (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Diltiazem (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Diltiazem (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Diltiazem (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Diltiazem (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Diltiazem (oral) Administration in the drug label.
Monitoring
There is limited information regarding Diltiazem (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Diltiazem (oral) and IV administrations.
Overdosage
There is limited information regarding Diltiazem (oral) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Diltiazem (oral) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Diltiazem (oral) Mechanism of Action in the drug label.
Structure
There is limited information regarding Diltiazem (oral) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Diltiazem (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Diltiazem (oral) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Diltiazem (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Diltiazem (oral) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Diltiazem (oral) How Supplied in the drug label.
Storage
There is limited information regarding Diltiazem (oral) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Diltiazem (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Diltiazem (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Diltiazem (oral) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Diltiazem sandbox interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Diltiazem (oral) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Diltiazem (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.