Clofibrate: Difference between revisions
Matt Pijoan (talk | contribs) m (Protected "Clofibrate": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
||
| Line 32: | Line 32: | ||
'''Associate Editor:''' {{CZ}} | '''Associate Editor:''' {{CZ}} | ||
==[[Clofibrate (patient information)|For patient information, click here]]== | ==[[Clofibrate (patient information)|For patient information, click here]]== | ||
| Line 218: | Line 218: | ||
{{Lipid modifying agents}} | {{Lipid modifying agents}} | ||
[[Category:Fibrates]] | [[Category:Fibrates]] | ||
[[Category:Prodrugs]] | [[Category:Prodrugs]] | ||
Revision as of 23:58, 8 August 2012
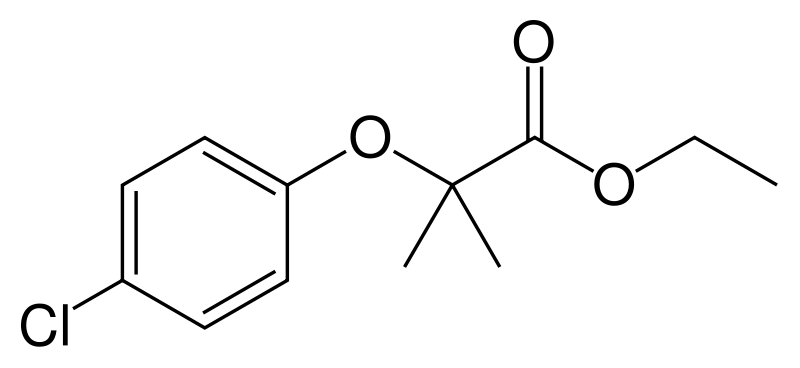 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Variable, 92–97% at therapeutic concentrations |
| Metabolism | Hydrolyzed to clofibric acid; hepatic glucuronidation |
| Elimination half-life | Highly variable; average 18–22 hours. Prolonged in renal failure |
| Excretion | Renal, 95 to 99% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C12H15ClO3 |
| Molar mass | 242.698 g/mol |
|
WikiDoc Resources for Clofibrate |
|
Articles |
|---|
|
Most recent articles on Clofibrate |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Clofibrate at Clinical Trials.gov Clinical Trials on Clofibrate at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Clofibrate
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Clofibrate Discussion groups on Clofibrate Patient Handouts on Clofibrate Directions to Hospitals Treating Clofibrate Risk calculators and risk factors for Clofibrate
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Clofibrate |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
| Cardiology Network |
 Discuss Clofibrate further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor: Cafer Zorkun, M.D., Ph.D. [2]
For patient information, click here
Overview
Clofibrate is a fibrate. It is used in the treatment of cardiovascular disease.
Generic Available
Yes
Use
Adjunct to dietary therapy in the management of hyperlipidemias associated with high triglyceride levels (types III, IV, V); primarily lowers triglycerides and very low density lipoprotein
Pregnancy Risk Factor
C
Lactation
Excretion in breast milk unknown/contraindicated
Contraindications
Hypersensitivity to clofibrate or any component of the formulation; significant hepatic or renal dysfunction; primary biliary cirrhosis
Warnings/Precautions
Possible increased risk of malignancy and cholelithiasis. No evidence of cardiovascular mortality benefit. Anemia and leukopenia have been reported. Elevations in serum transaminases can be seen. Discontinue if lipid response is not seen. Use with caution in peptic ulcer disease. Flu-like symptoms may occur. Be careful in patient selection; this is not a first- or second-line choice. Other agents may be more suitable.
Adverse Reactions
Frequency not defined
Common: Gastrointestinal: Nausea, diarrhea
Less common:
Central nervous system: Headache, dizziness, fatigue
Gastrointestinal: Vomiting, loose stools, heartburn, flatulence, abdominal distress, epigastric pain
Neuromuscular & skeletal: Muscle cramping, aching, weakness, myalgia
Frequency unknown
Central nervous system: Fever
Cardiovascular: Chest pain, cardiac arrhythmia
Dermatologic: Rash, urticaria, pruritus, alopecia, toxic epidermal necrolysis, erythema multiforme, Stevens-Johnson syndrome; dry, brittle hair
Endocrine & metabolic: Polyphagia, gynecomastia, hyperkalemia
Gastrointestinal: Stomatitis, gallstones, pancreatitis, gastritis, peptic ulcer, weight gain
Genitourinary: Impotence, decreased libido
Hematologic: Leukopenia, anemia, eosinophilia, agranulocytosis, thrombocytopenic purpura
Hepatic: Hepatomegaly, jaundice, liver function tests increased
Local: Thrombophlebitis
Neuromuscular & skeletal: Myalgia, myopathy, myositis, arthralgia, rhabdomyolysis, increased creatinine phosphokinase (CPK), rheumatoid arthritis, tremor
Ocular: Photophobic
Renal: Dysuria, hematuria, proteinuria, renal toxicity (allergic), rhabdomyolysis-induced renal failure
Miscellaneous: Diaphoresis increased, flu-like syndrome, systemic lupus erythematosus
Overdosage/Toxicology Symptoms of overdose include nausea, vomiting, diarrhea, and GI distress. Treatment is supportive.
Drug Interactions
Substrate of CYP3A4 (minor); Inhibits CYP2A6 (weak); Induces CYP2B6 (weak), 2E1 (weak), 3A4 (weak)
Chlorpropamide: May increase risk of hypoglycemia.
Furosemide: Blood levels of furosemide and fibric acid derivatives (ie, clofibrate and fenofibrate) may be increased during concurrent dosing (particularly in hypoalbuminemia). Limited documentation; monitor for increased effect/toxicity.
HMG-CoA reductase inhibitors (atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, simvastatin) may increase the risk of myopathy and rhabdomyolysis. The manufacturer warns against the concomitant use. However, combination therapy with statins has been used in some patients with resistant hyperlipidemias (with great caution).
Insulin: Hypoglycemic effects may be potentiated by an unknown mechanism.
Probenecid may decrease the clearance of clofibrate.
Rifampin: Decreased clofibrate blood levels.
Sulfonylureas (including glyburide, glipizide): Hypoglycemic effects may be potentiated by an unknown mechanism.
Warfarin: Increased hypoprothrombinemic response; monitor INRs closely when clofibrate is initiated or discontinued.
Mechanism of Action
Mechanism is unclear but thought to reduce cholesterol synthesis and triglyceride hepatic-vascular transference
Pharmacodynamics/Kinetics
Absorption: Complete
Distribution: Vd: 5.5 L/kg; crosses placenta
Protein binding: 95%
Metabolism: Hepatic to an inactive glucuronide ester; intestinal transformation required to activate drug
Half-life elimination: 6-24 hours, significantly prolonged with renal impairment; Anuria: 110 hours
Time to peak, serum: 3-6 hours
Excretion: Urine (40% to 70%)
Dosage
Adults: Oral: 500 mg 4 times/day; some patients may respond to lower doses
Dosing interval in renal impairment:
Clcr >50 mL/minute: Administer every 6-12 hours
Clcr 10-50 mL/minute: Administer every 12-18 hours
Clcr<10 mL/minute: Avoid use
Hemodialysis: Elimination is not enhanced via hemodialysis; supplemental dose is not necessary
Administration
Administer with meals or milk if GI upset occurs.
Monitoring Parameters
Serum lipids, cholesterol and triglycerides, LFTs, CBC
Test Interactions
Increased creatine phosphokinase [CPK] (S); decreased alkaline phosphatase (S), cholesterol (S), glucose, uric acid (S)
Patient Education
Inform prescriber of all prescriptions, OTC medications, or herbal products you are taking, and any allergies you have. Do not take any new medication during therapy unless approved by prescriber. Take as directed. May take with food or milk to reduce stomach upset.
This drug may have to be taken long-term; ongoing follow-up is essential. May cause nausea, vomiting, or stomach upset (small, frequent meals, frequent mouth care, chewing gum, or sucking lozenges may help); headache, dizziness, fatigue (use care when driving or engaging in potentially hazardous tasks until response to drug is known); or muscle cramping or pain (if persistent, consult prescriber for analgesic).
Report chest pain, shortness of breath, irregular heartbeat, palpitations, severe stomach pain with persistent nausea and vomiting, persistent fever, sore throat, or unusual bleeding or bruising. Pregnancy/breast-feeding precautions: Inform prescriber if you are or intend to become pregnant. Do not breast-feed.
Nursing Implications
Monitor serum lipids, LFTs, CBC
Cardiovascular Considerations
Fibric acids decrease triglycerides (TGs) by 20% to 50%, and increase HDL-cholesterol (HDL-C) by 10% to 35%. They decrease LDL-cholesterol (LDL-C) by 5% to 20%, however, LDL-C actually may increase by 10% to 30% when fibrates are initiated in patients with high TGs (>400 mg/dL).
Dental Health: Effects on Dental Treatment
Key adverse event(s) related to dental treatment: Stomatitis.
Dental Health: Vasoconstrictor/Local Anesthetic Precautions No information available to require special precautions
Mental Health: Effects on Mental Status
May cause sedation or dizziness
Mental Health: Effects on Psychiatric Treatment Rare reports of agranulocytosis; use caution with clozapine and carbamazepine
Dosage Forms
Capsule: 500 mg
References
"Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III)," JAMA , 2001, 285(19):2486-97.
Mahley RW and Bersot TP, "Drug Therapy for Hypercholesterolemia and Dyslipidemia," Goodman and Gilman's The Pharmacological Basis of Therapeutics , 10th ed, Hardman JE and Limbird LE, eds, New York, NY: McGraw-Hill, 2001, 993-5.
"WHO Cooperative Trial on Primary Prevention of Ischaemic Heart Disease With Clofibrate to Lower Serum Cholesterol: Final Mortality Follow-up. Report of the Committee of Principal Investigators," Lancet , 1984, 2(8403):600-4.
International Brand Names
Arterioflexin® (AT) Atromid® (HK) Atromidin® (LU) Claripex (CA) Clof® (CH) Clofibrat Tripharma® (CH) Elpi® (AR) Levatrom® (IL) Lipilim® (HK) Novo-Fibrate (CA)
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Fibrates
- Prodrugs
- Cardiology
- Drugs