Chagas disease: Difference between revisions
| Line 42: | Line 42: | ||
==Treatment== | ==Treatment== | ||
[[Chagas disease medical therapy|Medical therapy]] | [[Chagas disease surgery|Surgical options]] | [[Chagas disease primary prevention|Primary prevention]] | [[Chagas disease secondary prevention|Secondary prevention]] | [[Chagas disease cost-effectiveness of therapy|Financial costs]] | [[Chagas disease future or investigational therapies|Future therapies]] | [[Chagas disease medical therapy|Medical therapy]] | [[Chagas disease surgery|Surgical options]] | [[Chagas disease primary prevention|Primary prevention]] | [[Chagas disease secondary prevention|Secondary prevention]] | [[Chagas disease cost-effectiveness of therapy|Financial costs]] | [[Chagas disease future or investigational therapies|Future therapies]] | ||
==Prevention== | ==Prevention== | ||
[[Image:Triatoma infestans.jpg|thumb|Vector insect ''[[Triatoma infestans]]'' (Kissing Bug)]] | [[Image:Triatoma infestans.jpg|thumb|Vector insect ''[[Triatoma infestans]]'' (Kissing Bug)]] | ||
Revision as of 15:20, 27 January 2012
| Chagas disease | |
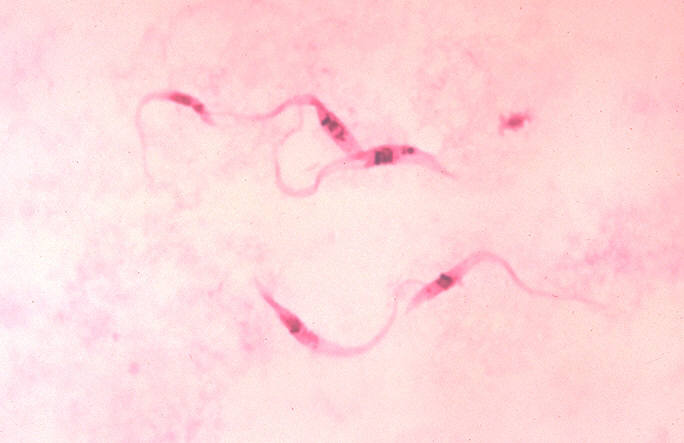 | |
|---|---|
| Photomicrograph of Giemsa-stained Trypanosoma cruzi crithidia (CDC) | |
| ICD-10 | B57 |
| ICD-9 | 086 |
| DiseasesDB | 13415 |
| MedlinePlus | 001372 |
| MeSH | D014355 |
For patient information click here
|
Chagas disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Chagas disease On the Web |
|
American Roentgen Ray Society Images of Chagas disease |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Historical Perspective
Pathophysiology
Epidemiology & Demographics
Risk Factors
Screening
Causes
Differentiating Chagas disease
Complications & Prognosis
Diagnosis
History and Symptoms | Physical Examination | Staging | Laboratory tests | Electrocardiogram | X Rays | CT | MRI Echocardiography or Ultrasound | Other images | Alternative diagnostics
Treatment
Medical therapy | Surgical options | Primary prevention | Secondary prevention | Financial costs | Future therapies
Prevention

A reasonably effective vaccine was developed in Ribeirão Preto in the 1970s, using cellular and subcellular fractions of the parasite, but it was found economically unfeasible. More recently, the potential of DNA vaccines for immunotherapy of acute and chronic Chagas' disease is being tested by several research groups.
Prevention is centered on fighting the vector (Triatoma) by using sprays and paints containing insecticides (synthetic pyrethroids), and improving housing and sanitary conditions in the rural area. For urban dwellers, spending vacations and camping out in the wilderness or sleeping at hostels or mud houses in endemic areas can be dangerous, a mosquito net is recommended. If the traveller intends to travel to the area of prevalence, he/she should get information on endemic rural areas for Chagas' disease in traveller advisories, such as the CDC.
In most countries where Chagas' disease is endemic, testing of blood donors is already mandatory, since this can be an important route of transmission. The United States FDA has recently licensed a test for antibodies against T. cruzi for use on blood donors but has not yet mandated its use. The AABB recommends that past recipients of blood components from donors found to be infected be notified and themselves tested. In the past, donated blood was mixed with 0,25 g/L of gentian violet successfully to kill the parasites. Early detection and treatment of new cases, including mother-to-baby cases, will also help reduce the burden of disease.
With all these measures, some landmarks were achieved in the fight against Chagas' disease in Latin America: a reduction by 72% of the incidence of human infection in children and young adults in the countries of the Initiative of the Southern Cone, and at least two countries (Uruguay, in 1997, and Chile, in 1999), were certified free of vectorial and transfusional transmission. In Brazil, with the largest population at risk, 10 out of the 12 endemic states were also certified free.
Some stepstones of vector control:
- A yeast trap has been tested for monitoring infestations of certain species of the bugs:"Performance of yeast-baited traps with Triatoma sordida, Triatoma brasiliensis, Triatoma pseudomaculata, and Panstrongylus megistus in laboratory assays."[1]
- Promising results were gained with the treatment of vector habitats with the fungus Beauveria bassiana, (which is also in discussion for malaria- prevention):"Activity of oil-formulated Beauveria bassiana against Triatoma sordida in peridomestic areas in Central Brazil."[2]
- Targeting the symbionts of Triatominae through paratransgenesis.[3]
See also
- Tropical disease
- Drugs for Neglected Diseases Initiative
- Distinguish from: Chaga mushroom
Notes
- ↑ Pires HH, Lazzari CR, Diotaiuti L, Lorenzo MG. "Performance of yeast-baited traps with Triatoma sordida, Triatoma brasiliensis, Triatoma pseudomaculata, and Panstrongylus megistus in laboratory assays." Rev Panam Salud Publica. 2000 Jun;7(6):384-8. PMID 10949899
- ↑ Luz C, Rocha LF, Nery GV, Magalhaes BP, Tigano MS. "Activity of oil-formulated Beauveria bassiana against Triatoma sordida in peridomestic areas in Central Brazil." Mem Inst Oswaldo Cruz. 2004 Mar;99(2):211-8. PMID 15250478 Online.
- ↑ "PubMed Search on Triatominae symbiosis".
References
- CDC, Division of Parasitic Diseases. Chagas Disease Fact Sheet. (23 September 2004). Accessed 24 September 2006.
- Dumonteil E, Escobedo-Ortegon J, Reyes-Rodriguez N, Arjona-Torres A, Ramirez-Sierra M (2004). "Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice". Infect Immun. 72 (1): 46–53. PMID 14688079.
- CDC, Division of Parasitic Diseases.Chagas Disease(23 October 2007). Accessed 16 August 2007.
- CDC, Division of Parasitic Diseases.Chagas Disease Epidemiology.(23 October 2007). Accessed 16 August 2007.
- CDC, Division of Parasitic Diseases. Treatment(23 October 2007). Accessed 16 August 2007.
Further reading
- Coutinho M (1999). "Ninety years of Chagas disease: a success story at the periphery". Soc Stud Sci. 29 (4): 519–49. PMID 11623933.
- Dias J, Silveira A, Schofield C (2002). "The impact of Chagas disease control in Latin America: a review". Mem Inst Oswaldo Cruz. 97 (5): 603–12. PMID 12219120.
- Kropf S, Azevedo N, Ferreira L (2003). "Biomedical research and public health in Brazil: the case of Chagas' disease (1909–50)". Soc Hist Med. 16 (1): 111–29. PMID 14598820.
- "International Symposium to commemorate the 90th anniversary of the discovery of Chagas disease (Rio de Janeiro, April 11–16, 1999)". Memorias do Instituto Oswaldo Cruz. 94 (Suppl. I). 1999.
- Moncayo A. "Progress towards interruption of transmission of Chagas disease". Mem Inst Oswaldo Cruz. 94 Suppl 1: 401–4. PMID 10677765.
- Prata A. "Evolution of the clinical and epidemiological knowledge about Chagas disease 90 years after its discovery". Mem Inst Oswaldo Cruz. 94 Suppl 1: 81–8. PMID 10677694.
- Franco-Paredes C (2007). "Chagas disease: an impediment in achieving the Millennium Development Goals in Latin America". BMC International Health and Human Rights. 7: 7. PMID 17725836.
- Kevin M. Tyler & Michael A. Miles (ed.). World Class Parasites. Volume 7: American Trypanosomiasis. Kluwer Academic Publishers. ISBN 1-4020-7323-2. Amazon review
External links
- VCU Virtual Parasite Project
- All About Chagas Disease, Chagas' disease Information & Prevention, Identification, also in Spanish.
- Chagaspace, also in Spanish.
- ChagMex: Database on-line. UNAM-Instituto de Biología.
- Chagas Disease. PanAmerican Health Organization.
- Disease Information. American Trypanosomiasis or Chagas' Disease. Travel Medicine Program. Health Canada.
- Links to Chagas' Disease pictures (Hardin MD/Univ of Iowa)
- Link to "The Kiss of Death". An anthropological view of Chagas' disease (Joseph Bastien/Univ of Texas at Arlington).
Recent news and events
- Chagas' disease parasite found in desert blood samples
- Chagas Control in the Southern Cone Countries: History of an International Initiative, 1991/2001, PAHO. (Full text e-book)
- Genome Sequencing Project
- Parasites' genetic code 'cracked' From BBC
- Catholic Relief Services. Housing Improvement and Chagas' Disease Prevention Project
- 2006: Nature.com / Scott M. Landfear: Flagella are whip-like structures that power the movement of certain cells. Analysis of a single-cell parasite, the African trypanosome, reveals that flagella are also essential for viability in this organism. (restricted commercial access now)
- Science Magazine Search Results: Chagas
Template:Protozoal diseases Template:Link FA ar:شاجاس ca:Malaltia de Chagas de:Chagas-Krankheit it:Malattia di Chagas lt:Čagaso liga ms:Penyakit Cagas nl:Ziekte van Chagas sv:Chagas sjukdom