Cavernous angioma
Template:DiseaseDisorder infobox
|
Cavernous angioma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Cavernous angioma On the Web |
|
American Roentgen Ray Society Images of Cavernous angioma |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Synonyms and keywords: Cerebral cavernous malformation; CCM; cavernous hemangioma, cavernous haemangioma; cavernoma
Incidence of occurrence
The incidence in the general population is between 0.1–0.5%, and clinical symptoms typically appear between 30 to 50 years of age. Once thought to be strictly congenital, these vascular lesions have been found to occur de novo.
Diagnosis
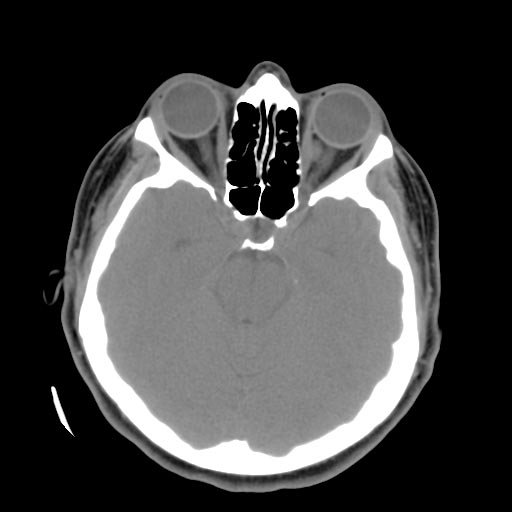
Diagnosis is most commonly made accidentally by routine magnetic resonance imaging (MRI) screening, but not all MRI exams are created equal. It is paramount that the patient request a gradient-echo sequence in order to unmask small or punctate lesions which may otherwise remain undetected. These lesions are also more conspicuous on FLAIR imaging compared to standard T2 weighing. FLAIR imaging is different from Gradient sequences, rather, it is similar to T2 weighing but suppresses free-flowing fluid signal. Sometimes quiescent CCMs can be revealed as incidental findings during MRI exams ordered for other reasons.
Sometimes the lesion appearance imaged by MRI remains inconclusive. Consequently neurosurgeons will order a cerebral angiogram or magnetic resonance angiogram (MRA). Since CCMs are low flow lesions (they are hooked into the venous side of the circulatory system), they will be angiographically occult (invisible). If a lesion is discernible via angiogram in the same location as in the MRI, then an arteriovenous malformation (AVM) becomes the primary concern.
History and Symptoms
Clinical symptoms of this disease include recurrent headaches, focal neurological deficits, hemorrhagic stroke, and seizures, but CCM can also be asymptomatic.
This disease is characterized by grossly dilated blood vessels with a single layer of endothelium and an absence of neuronal tissue within the lesions. These thinly-walled vessels resemble sinusoidal cavities filled with stagnant blood. Blood vessels in patients with CCM can range from a few millimeters to several centimeters in diameter. CCM lesions commonly resemble raspberries in external structure.
Many patients live their whole life without knowing they have a cerebral cavernous malformation. Other patients can have severe symptoms like seizures, headaches, paralysis, bleeding in the brain (cerebral hemorrhage, or hemorrhagic stroke), and even death. The nature and severity of the symptoms depend on the lesion's location in the brain. Approximately 70% of these lesions occur in the supratentorial region of the brain; the remaining 30% occur in the infratentorial region.
Physical Examination
Skin
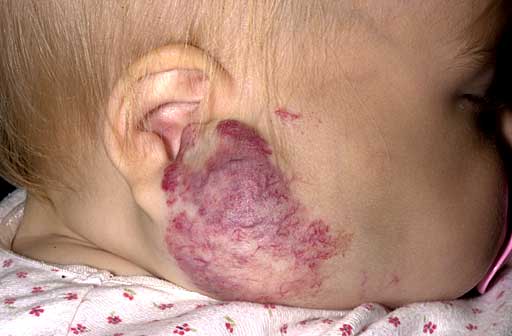
CCMs & venous angiomas
In up to 30% there is a coincidence of CCM with a venous angioma, also known as a developmental venous anomaly (DVA). These lesions appear either as enhancing linear blood vessels or caput medusae, a radial orientation of small vessels that resemble the hair of Medusa from Greek mythology. These lesions are thought to represent developmental anomalies of normal venous drainage. These lesions should not be removed, as reports of venous infarcts have been reported. When found in association with a CCM that needs resection, great care should be taken not to disrupt the angioma.
Left orbital cavernous hemangioma
Familial forms
Familial forms of CCM occur at three known genetic loci. The gene for CCM1 encodes KRIT1 (krev interaction trapped 1) and has been found to bind to ICAP1alpha (integrin cytoplasmic domain associated protein alpha), a beta1 integrin associated protein. The gene for CCM2 encodes a novel protein named "malcavernin" that contains a phosphotyrosine (PTB) binding domain. The exact biological function of CCM2 is not clear. Recently, it has been shown that CCM1 and CCM2 proteins as well as ICAP1alpha form a macromolecular complex in the cell. In addition, it appears that CCM2 protein may function as a scaffolding protein for MAP kinases that are essential in p38 activation responding to osmotic stress including MEKK3 and MKK3. It also binds to Rac and actin. Therefore, CCM2 protein is also called OSM (osmosensing scaffold for MEKK3). The CCM3 gene was the most recent CCM gene identified. CCM3 was known as PDCD10 (programmed cell death 10), which was initially identified as a gene that is up-regulated during the induction of apoptosis (cell death) in TF-1, a human myeloid cell line. The precise role of the PDCD10 protein in the CCM pathway that has been established to this point has not yet been determined. Research is ongoing to determine the function and properties of all three CCM gene products as well as the reaction pathways in which they are involved.
Mutations in these three genes account for 70 to 80 percent of all cases of cerebral cavernous malformations. The remaining 20 to 30 percent of cases may be due to other, still unidentified, genes.