17 alpha-hydroxylase deficiency pathophysiology: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
* An expected second 17,20-lyase reaction (17-hydroxyprogesterone → [[androstenedione]]) is mediated so inefficiently in humans as to be of no known significance. | * An expected second 17,20-lyase reaction (17-hydroxyprogesterone → [[androstenedione]]) is mediated so inefficiently in humans as to be of no known significance. | ||
* The hydroxylase reactions are part of the synthetic pathway to cortisol as well as sex steroids, but the lyase reaction is only necessary for sex steroid synthesis. | * The hydroxylase reactions are part of the synthetic pathway to cortisol as well as sex steroids, but the lyase reaction is only necessary for sex steroid synthesis. | ||
* The dual enzyme activities were for many decades assumed to represent two entirely different genes and enzymes. Thus, medical textbooks and nosologies until quite recently described two different diseases: | * The dual enzyme activities were for many decades assumed to represent two entirely different genes and enzymes. Thus, medical textbooks and nosologies until quite recently described two different diseases: 17α-hydroxylase deficient congenital adrenal hyperplasia, and a distinct and even rarer defect of sex steroid synthesis termed 17,20-lyase deficiency (which is not a form of congenital adrenal hyperplasia). In the last decade, it has become clearer that the two diseases are different forms of defects of the same gene. However, the clinical features of the two types of impairment are distinct enough that they are described separately in the following sections. | ||
===Mineralocorticoid Effects=== | ===Mineralocorticoid Effects=== | ||
* The adrenal cortex is hyperplastic and overstimulated, with no impairment of the mineralocorticoid pathway. Consequently, levels of deoxycorticosterone, [[corticosterone]], and 18-deoxycorticosterone are elevated. Although these precursors of [[aldosterone]] are weaker mineralocorticoids, the extreme elevations usually provide enough volume expansion, blood pressure elevation, and potassium depletion to suppress [[renin]] and aldosterone production. Some persons with 17α-hydroxylase deficiency develop [[hypertension]] in infancy, and nearly 90% do so by late childhood. The low-renin [[hypertension]] is often accompanied by [[hypokalemia]] due to urinary potassium wasting and [[metabolic alkalosis]]. These features of mineralocorticoid excess are the major clinical clue distinguishing the more complete 17α-hydroxylase deficiency from the 17,20-lyase deficiency, which only affects the sex steroids. Treatment with glucocorticoid suppresses ACTH, returns mineralocorticoid production toward normal, and lowers blood pressure. | * The adrenal cortex is hyperplastic and overstimulated, with no impairment of the mineralocorticoid pathway. Consequently, levels of deoxycorticosterone, [[corticosterone]], and 18-deoxycorticosterone are elevated. Although these precursors of [[aldosterone]] are weaker mineralocorticoids, the extreme elevations usually provide enough volume expansion, blood pressure elevation, and potassium depletion to suppress [[renin]] and aldosterone production. Some persons with 17α-hydroxylase deficiency develop [[hypertension]] in infancy, and nearly 90% do so by late childhood. The low-renin [[hypertension]] is often accompanied by [[hypokalemia]] due to urinary potassium wasting and [[metabolic alkalosis]]. These features of mineralocorticoid excess are the major clinical clue distinguishing the more complete 17α-hydroxylase deficiency from the 17,20-lyase deficiency, which only affects the sex steroids. Treatment with glucocorticoid suppresses ACTH, returns mineralocorticoid production toward normal, and lowers blood pressure. | ||
Revision as of 15:56, 4 February 2016
|
Congenital adrenal hyperplasia due to 17 alpha-hydroxylase deficiency Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
17 alpha-hydroxylase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of 17 alpha-hydroxylase deficiency pathophysiology |
|
Risk calculators and risk factors for 17 alpha-hydroxylase deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Overview
Pathogenesis
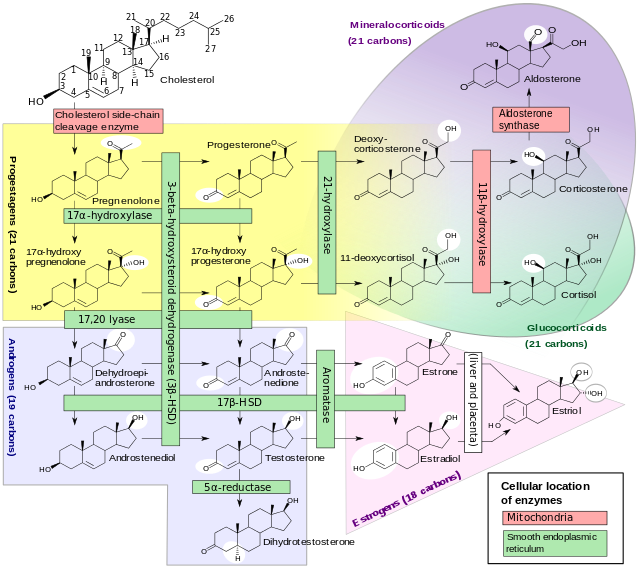
- Congenital adrenal hyperplasia due to 17 alpha-hydroxylase deficiency produces decreased synthesis of both cortisol and sex steroids, with resulting increase in mineralocorticoid production. Thus, common symptoms include mild hypocortisolism, ambiguous genitalia in genetic males or failure of the ovaries to function at puberty in genetic females, and hypertension (respectively). This form of CAH results from deficiency of the enzyme 17α-hydroxylase (also called CYP17A1).
- Congenital adrenal hyperplasia due to 17 alpha-hydroxylase deficiency accounts for less than 5% of the cases of congenital adrenal hyperplasia. 17α-hydroxylase deficiency impairs the efficiency of cortisol synthesis, resulting in high levels of ACTH secretion and hyperplasia of the adrenal glands.
- Clinical effects of this condition include overproduction of mineralocorticoids and deficiency of prenatal and pubertal sex steroids. CYP17A1 functions in steroidogenesis, where it converts pregnenolone and progesterone to their 17-hydroxy forms. The enzyme itself is attached to the smooth endoplasmic reticulum of the steroid-producing cells of the adrenal cortex and gonads. CYP17A1 functions as both a 17α-hydroxylase and a 17,20-lyase. The dual activities mediate three key transformations in cortisol and sex steroid synthesis:
- As 17α-hydroxylase it mediates pregnenolone → 17-hydroxypregnenolone and progesterone → 17-hydroxyprogesterone.
- As 17,20-lyase it mediates 17-hydroxypregnenolone → DHEA.
- An expected second 17,20-lyase reaction (17-hydroxyprogesterone → androstenedione) is mediated so inefficiently in humans as to be of no known significance.
- The hydroxylase reactions are part of the synthetic pathway to cortisol as well as sex steroids, but the lyase reaction is only necessary for sex steroid synthesis.
- The dual enzyme activities were for many decades assumed to represent two entirely different genes and enzymes. Thus, medical textbooks and nosologies until quite recently described two different diseases: 17α-hydroxylase deficient congenital adrenal hyperplasia, and a distinct and even rarer defect of sex steroid synthesis termed 17,20-lyase deficiency (which is not a form of congenital adrenal hyperplasia). In the last decade, it has become clearer that the two diseases are different forms of defects of the same gene. However, the clinical features of the two types of impairment are distinct enough that they are described separately in the following sections.
Mineralocorticoid Effects
- The adrenal cortex is hyperplastic and overstimulated, with no impairment of the mineralocorticoid pathway. Consequently, levels of deoxycorticosterone, corticosterone, and 18-deoxycorticosterone are elevated. Although these precursors of aldosterone are weaker mineralocorticoids, the extreme elevations usually provide enough volume expansion, blood pressure elevation, and potassium depletion to suppress renin and aldosterone production. Some persons with 17α-hydroxylase deficiency develop hypertension in infancy, and nearly 90% do so by late childhood. The low-renin hypertension is often accompanied by hypokalemia due to urinary potassium wasting and metabolic alkalosis. These features of mineralocorticoid excess are the major clinical clue distinguishing the more complete 17α-hydroxylase deficiency from the 17,20-lyase deficiency, which only affects the sex steroids. Treatment with glucocorticoid suppresses ACTH, returns mineralocorticoid production toward normal, and lowers blood pressure.
Glucocorticoid effects
- Although production of cortisol is inefficient enough to normalize ACTH, the 50-100-fold elevations of corticosterone have enough weak glucocorticoid activity to prevent glucocorticoid deficiency and adrenal crisis.
Sex steroid effects
- Genetic XX females affected by 17α-hydroxylase deficiency are born with normal female internal and external anatomy. At the expected time of puberty, neither the adrenals nor the ovaries can produce sex steroids, so neither breast development nor pubic hair appear. Investigation of delayed puberty yields elevated gonadotropins and normal karyotype, while imaging confirms the presence of ovaries and an infantile uterus. Discovery of hypertension and hypokalemic alkalosis usually suggests the presence of one of the proximal forms of CAH, and the characteristic mineralocorticoid elevations confirm the specific diagnosis.
- A few milder forms of this deficiency in genetic females have allowed relatively normal breast development and irregular menstruation. Evidence suggests that only 5% of normal enzyme activity may be enough to allow at least the physical changes of female puberty, if not ovulation and fertility. In these girls, the elevated blood pressure was the primary clinical problem.
- 17α-Hydroxylase deficiency in genetic males (XY) results in moderate to severe reduction of fetal testosterone production by both adrenals and testes. Undervirilization is variable and sometimes complete. The appearance of the external genitalia ranges from normal female to ambiguous to mildly underdeveloped male. The most commonly described phenotype is a small phallus, perineal hypospadias, small blind pseudovaginal pouch, and intra-abdominal or inguinal testes. Wolffian duct derivatives are hypoplastic or normal, depending on degree of testosterone deficiency. Some of those with partial virilization develop gynecomastia at puberty even though masculinization is reduced. The presence of hypertension in the majority distinguishes them from other forms of partial androgen deficiency or insensitivity. Fertility is impaired in those with more than minimal testoserone deficiency.
17,20-Lyase deficiency
- A very small number of people have reportedly had an abnormal allele that resulted primarily in a reduction of 17,20-lyase activity, rather than both the hydroxylase and lyase activities as described above. In these people the defect had the effect of an isolated impairment of sex steroid (e.g., DHEA in the adrenal, but also gonadal testosterone and estrogens) synthesis, whereas mineralocorticoid (e.g., aldosterone) and glucocorticoid (e.g., cortisol) levels remain normal.
- Normal aldosterone level can be attributed to the fact that aldosterone is independent of hypothalamus-pituitary axis feedback system, being mainly controlled by the level of serum potassium. Because of the normal aldosterone level, hypertension is not expected.
- Normal cortisol level can be explained by the strong negative feedback mechanism of cortisol on hypothalamus-pituitary axis system. That is, in the beginning, 17,20-lyase deficiency will block synthesis of sex steroid hormones, forcing the pathways to produce more cortisol. However, the initial excess of cortisol is rapidly corrected by negative feedback mechanism—high cortisol decreases secretion of adrenocorticotropic hormone (ACTH) from zona fasciculata of adrenal gland. Thus, there is no mineralocorticoid overproduction. Also, there is no adrenal hyperplasia.
- It has also been observed in patients that the adrenocorticotropic hormone (ACTH) level remains in the normal range. The reason for this is still unclear.
- The sex steroid deficiency produces effects similar to 17α-hydroxylase deficiency. Severely affected genetic females (XX) are born with normal internal and external genitalia and there are no clues to abnormality until adolescence, when both the androgenic and estrogenic signs (e.g., breasts and pubic hair) of puberty fail to occur. Gonadotropins are high and the uterus infantile in size. The ovaries may contain enlarged follicular cysts, and ovulation may not occur even after replacement of estrogen.
Genetics
- Congenital adrenal hyperplasia due to 17α-hydroxylase deficiency is inherited in an autosomal recessive manner. The most common abnormal alleles of this condition impair both the 17α-hydroxylase activity and the 17,20-lyase activity of CYP17A1.

Gross Pathology
- On gross pathology the following changes are noted:
- Thickening of adrenal gland[1]
- Cerebriform appearance
Microscopic Pathology
- On microscopic pathology the following changes are noted:
- Diffuse cortical hyperplasia
- Zona reticularis is markedly hyperplastic
- Lipid depleted cortical cells
References
- ↑ Adrenocortical hyperplasia. American urological association (2016). https://www.auanet.org/education/modules/pathology/adrenal-gland/hyperplasia.cfm Accessed on January 28, 2016