17-Hydroxyprogesterone: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{drugbox | | {{drugbox | | ||
| IUPAC_name = 17-Hidroxiprogesterona3D.png | | IUPAC_name =17-Hidroxiprogesterona3D.png | ||
| image =17-Hydroxyprogesterone.png | | image =17-Hydroxyprogesterone.png | ||
| CAS_number = | | CAS_number = | ||
Revision as of 13:50, 15 April 2015
 | |
| Pharmacokinetic data | |
|---|---|
| Metabolism | Liver |
| Identifiers | |
| |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
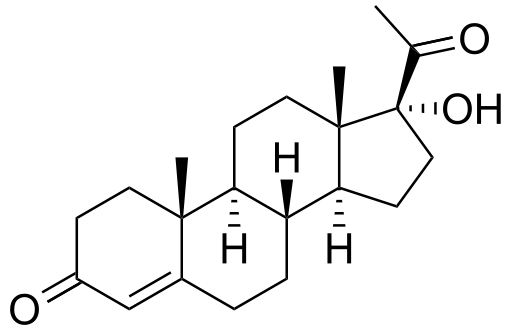
| Formula | C21H30O3 |
| Molar mass | 330.22 |
| Melting point | 219−220 °C (−145 °F) |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
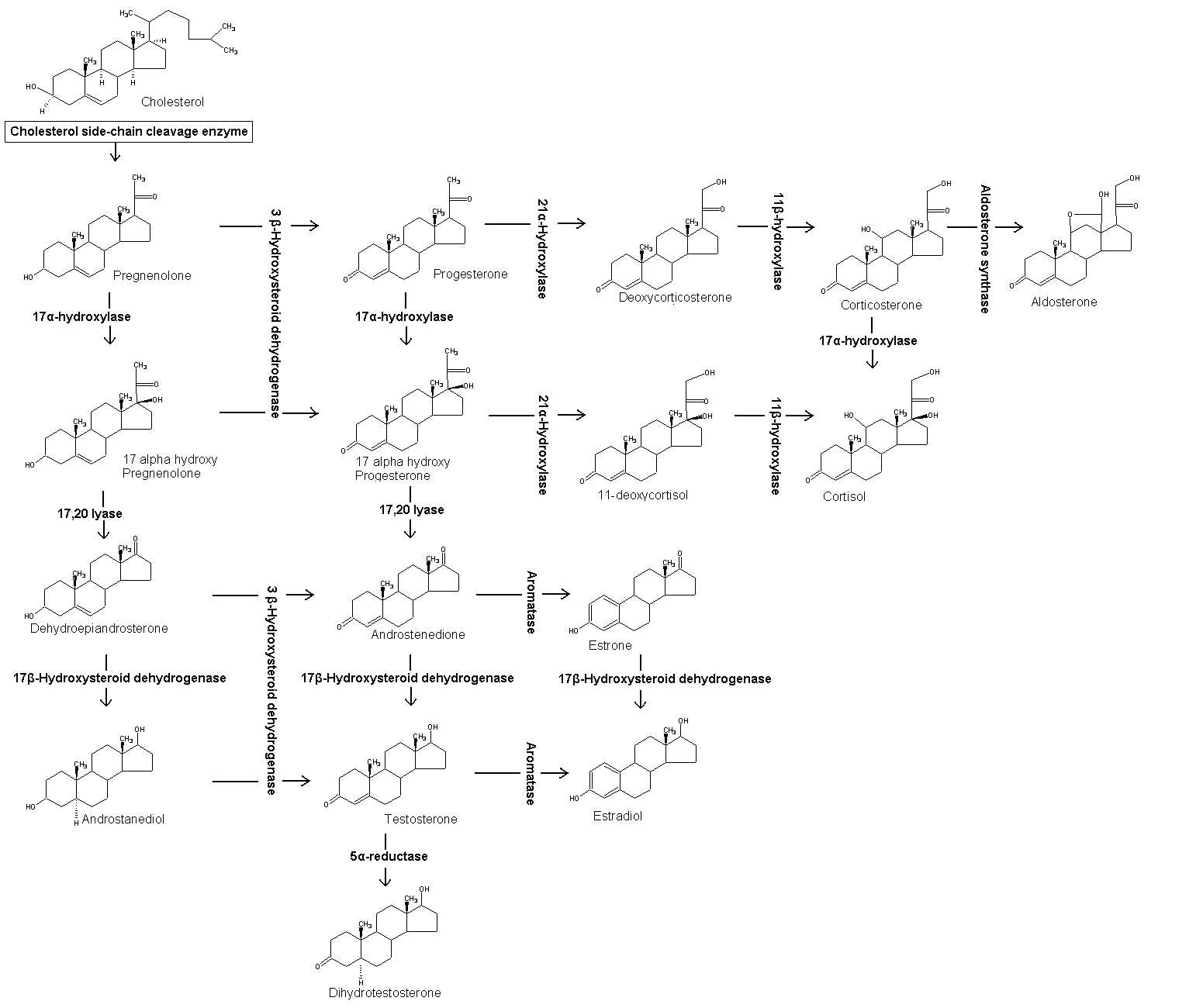
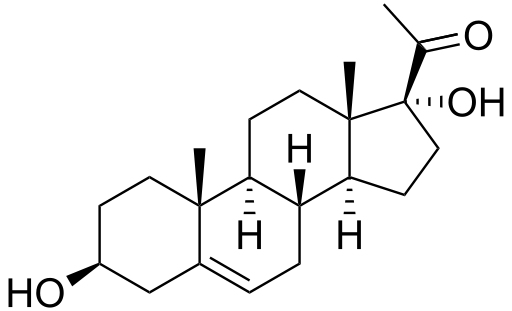
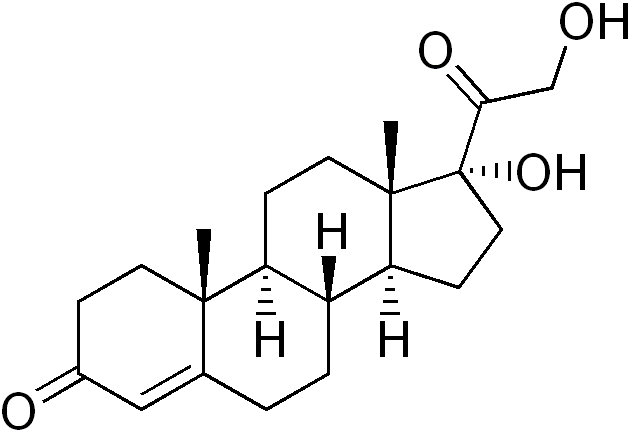
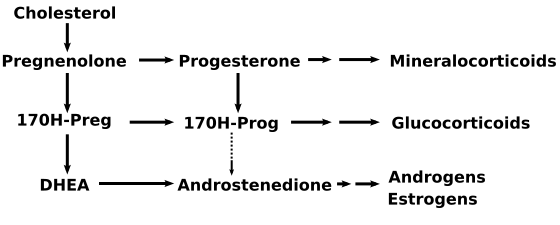
17-Hydroxyprogesterone (17-OH progesterone or 17OHP) is a C-21 steroid hormone produced in the synthesis of glucocorticoids and sex steroids.
As a hormone, 17OHP also interacts with the progesterone receptor.
Production
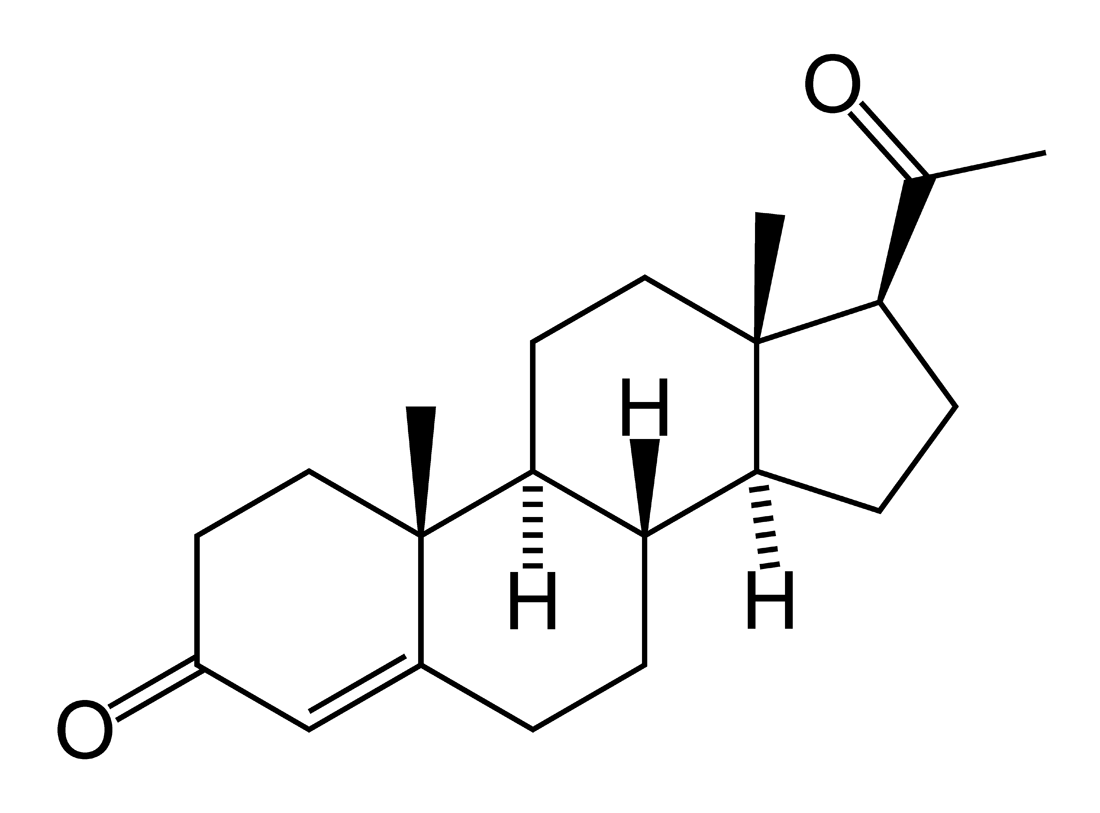
It is derived from progesterone via 17-hydroxylase, a P450c17 enzyme, or from 17-hydroxypregnenolone via 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase.
17-Hydroxyprogesterone is a natural progestin and in pregnancy increases in the third trimester primarily due to fetal adrenal production.
This hormone is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 15-70 ng/dl prior to ovulation, and 35-290 ng/dl during the luteal phase.
17-hydroxyprogesterone caproate
17-Hydroxyprogesterone is not the same compound as 17-hydroxyprogesterone caproate. 17-Hydroxyprogesterone caproate is a synthetic (artificial) hormone that is similar in structure to medroxyprogesterone acetate and megestrol acetate.
The terminology is confusing because 17P is used both to refer to the natural hormone and the artificial/synthetic hormone. It is preferable to refer to the synthetic hormone as 17-OHPC.
Clinical use
Measurements of levels of 17-hydroxyprogesterone are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase and 11β-hydroxylase, lead to a build-up of 17OHP. In contrast, the rare patient with 17α-hydroxylase deficiency will have very low or undetectable levels of 17OHP. 17OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but not 17OHP is also contributed by the placenta.
The application of 17OHP has been shown to be useful to delay premature labor in pregnancy.[1][2]
The use of 17-hydroxyprogesterone caproate in pregnancy to prevent preterm birth is not recommended without further study according to two authorities. A 2006 Cochrane Review concluded "...important maternal and infant outcomes have been poorly reported to date... information regarding the potential harms of progesterone therapy to prevent preterm birth is limited".[3] There was a similar conclusion from a review by Marc Keirse.[4] Three clinical studies of 250 mg/week of i.m. 17-hydroxyprogesterone caproate have all shown a trend for an increase in pregnancy loss due to miscarriage compared to placebo.[5][1][2][6] There has also been a study in rhesus monkeys in which all rhesus fetuses exposed to 1 and 10 times the human dose equivalent of 17-hydroxyprogesterone caproate died in utero.[7] Currently, 17-hydroxyprogesterone caproate is a category D progestin according to the FDA (evidence of fetal harm). There is speculation that the castor oil in the 17-hydroxyprogesterone caproate formulation may not be beneficial for pregnancy.[8][9]
Additional images
References
- ↑ 1.0 1.1 Yemini M, Borenstein R, Dreazen, et al. Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 1985;151(5):574-7.PMID 3976757.
- ↑ 2.0 2.1 Meis PJ; Society for Maternal-Fetal Medicine. 17 hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol. 2005;105:1128-35.PMID 15863556.
- ↑ Dodd JM, Flenady V, Cincotta R, Crowther CA; The Cochrane Database of Systematic Reviews 2006 Issue 1
- ↑ Keirse MJNC. Progesterone and Preterm: Seventy Years of "Deja vu" or "Still To Be Seen"?. Birth, 2004 September; 31:3.
- ↑ Johnson JWC, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of premature labor. NEJM 1975 October. 293(14):675.
- ↑ Keirse MJNC, Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynecol 1990 February; 97:149.
- ↑ Hendrix AG, et al. Embriotoxicity of sex steroidal hormones in nonhuman primates: II. Hydroxyprogesterone caproate, estradiol valerate. Teratology 1987 February. 35 (1): 129.
- ↑ Duke University Medical Center, New England Journal of Medicine, correspondence, vol 349.
- ↑ Hauth JC, Gilstrap LC, Brekken AL, Hauth JM. The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol. 1983 May; 146(2): 187.
External links
- Pages with script errors
- Pages with non-numeric formatnum arguments
- Pages with broken file links
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Progestagens
- Steroids