Cefepime clinical pharmacology
Cefepime is an antibacterial agent belonging to the cephalosporin class of antibacterials with in vitro antibacterial activity against facultative Gram-positive and Gram-negative bacteria.
Pharmacokinetics
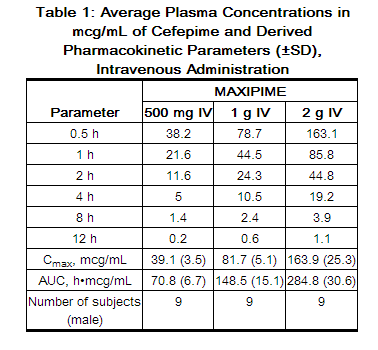
The average plasma concentrations of cefepime observed in healthy adult male volunteers (n=9) at various times following single 30-minute infusions (IV) of cefepime 500 mg, 1 g, and 2 g are summarized in Table 1. Elimination of cefepime is principally via renal excretion with an average (±SD) half-life of 2 (±0.3) hours and total body clearance of 120 (±8) mL/min in healthy volunteers. Cefepime pharmacokinetics are linear over the range 250 mg to 2 g. There is no evidence of accumulation in healthy adult male volunteers (n=7) receiving clinically relevant doses for a period of 9 days.
Absorption
The average plasma concentrations of cefepime and its derived pharmacokinetic parameters after intravenous (IV) administration are portrayed in Table 1.
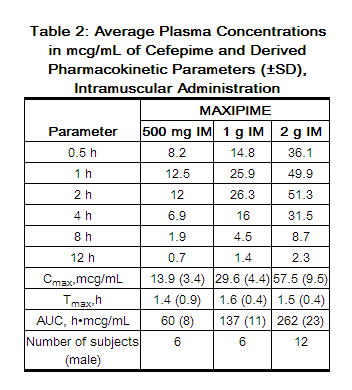
Following intramuscular (IM) administration, cefepime is completely absorbed. The average plasma concentrations of cefepime at various times following a single intramuscular injection are summarized in Table 2. The pharmacokinetics of cefepime are linear over the range of 500 mg to 2 g intramuscularly and do not vary with respect to treatment duration.
Distribution
The average steady-state volume of distribution of cefepime is 18 (±2) L. The serum protein binding of cefepime is approximately 20% and is independent of its concentration in serum.
Cefepime is excreted in human milk. A nursing infant consuming approximately 1000 mL of human milk per day would receive approximately 0.5 mg of cefepime per day. (See PRECAUTIONS: Nursing Mothers.)
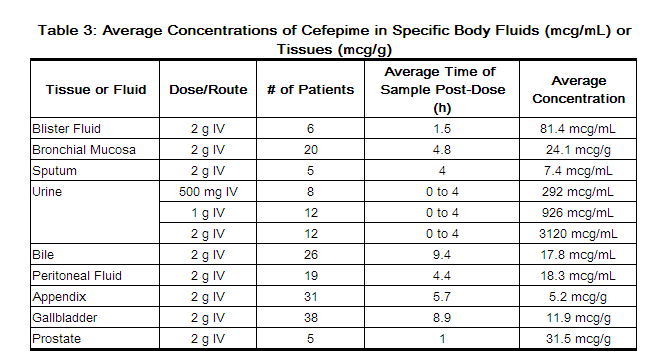
Concentrations of cefepime achieved in specific tissues and body fluids are listed in Table 3.