Hepatitis B laboratory tests
|
Hepatitis B |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis B laboratory tests On the Web |
|
American Roentgen Ray Society Images of Hepatitis B laboratory tests |
|
Risk calculators and risk factors for Hepatitis B laboratory tests |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor In Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
Lab Tests
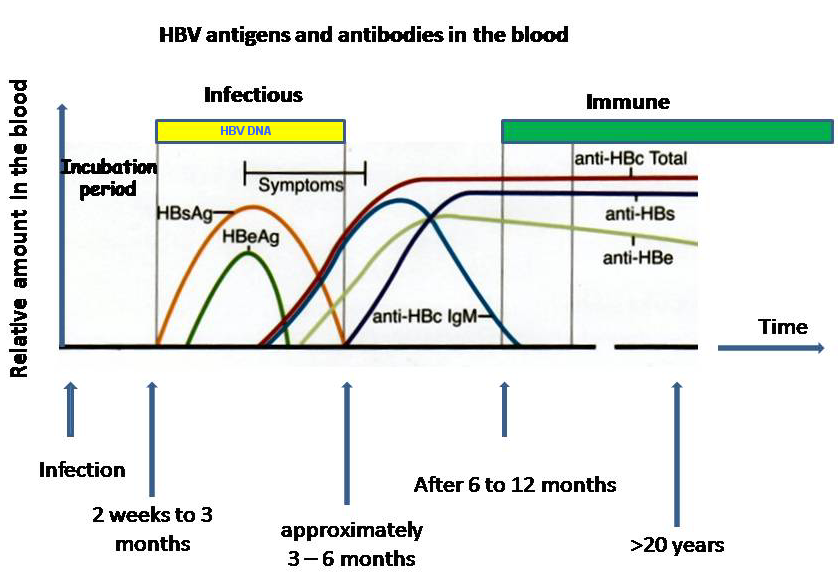
The tests, called assays, for detection of hepatitis B virus infection involve serum or blood tests that detect either viral antigens (proteins produced by the virus) or antibodies produced by the host. Interpretation of these assays is complex.[1]
The hepatitis B surface antigen (HBsAg) is most frequently used to screen for the presence of this infection. It is the first detectable viral antigen to appear during infection. However, early in an infection, this antigen may not be present and it may be undetectable later in the infection as it is being cleared by the host. The infectious virion contains an inner "core particle" enclosing viral genome. The icosahedral core particle is made of 180 or 240 copies of core protein, alternatively known as hepatitis B core antigen, or HBcAg. During this 'window' in which the host remains infected but is successfully clearing the virus, IgM antibodies to the hepatitis B core antigen (anti-HBc IgM) may be the only serological evidence of disease.
Shortly after the appearance of the HBsAg, another antigen named as the hepatitis B e antigen (HBeAg) will appear. Traditionally, the presence of HBeAg in a host's serum is associated with much higher rates of viral replication and enhanced infectivity; however, variants of the hepatitis B virus do not produce the 'e' antigen, so this rule does not always hold true. During the natural course of an infection, the HBeAg may be cleared, and antibodies to the 'e' antigen (anti-HBe) will arise immediately afterwards. This conversion is usually associated with a dramatic decline in viral replication.
If the host is able to clear the infection, eventually the HBsAg will become undetectable and will be followed by IgG antibodies to the hepatitis B surface antigen and core antigen, (anti-HBs and anti HBc IgG). A person negative for HBsAg but positive for anti-HBs has either cleared an infection or has been vaccinated previously.
Individuals who remain HBsAg positive for at least six months are considered to be hepatitis B carriers.[2] Carriers of the virus may have chronic hepatitis B, which would be reflected by elevated serum alanine aminotransferase levels and inflammation of the liver, as revealed by biopsy. Carriers who have seroconverted to HBeAg negative status, particularly those who acquired the infection as adults, have very little viral multiplication and hence may be at little risk of long-term complications or of transmitting infection to others.[3]
More recently, PCR tests have been developed to detect and measure the amount of viral nucleic acid in clinical specimens. These tests are called viral loads and are used to assess a person's infection status and to monitor treatment.[4]
The original assays for detection of hepatitis B virus infection involve serum or blood tests that detect either viral antigens (proteins produced by the virus) or antibodies produced by the host. Interpretation of these assays is complex. The table below is organized chronologically, from top to bottom:
| Antigens | Antibodies |
| The hepatitis B surface antigen (HBsAg[5]) is most frequently used to screen for the presence of this infection. It is the first detectable viral antigen to appear during infection with this virus; however, early in an infection, this antigen may not be present and it may be undetectable later in the infection as it is being cleared by the host. | - |
| The infectious virion contains an inner "core particle" enclosing viral genome. The icosahedral core particle is made of 180 or 240 copies of core protein, alternatively known as hepatitis B core antigen, or HBcAg[6] | During this 'window' in which the host remains infected but is successfully clearing the virus, IgM antibodies to the hepatitis B core antigen (anti-HBc IGM) may be the only serologic evidence of disease. |
| Shortly after the appearance of the HBsAg, another antigen named as the hepatitis B e antigen (HBeAg[7]) will appear. Traditionally, the presence of HBeAg in a host's serum is associated with much higher rates of viral replication; however, some variants of the hepatitis B virus do not produce the 'e' antigen at all, so this rule does not always hold true. | During the natural course of an infection, the HBeAg may be cleared, and antibodies to the 'e' antigen (anti-HBe) will arise immediately afterward. This conversion is usually associated with a dramatic decline in viral replication. |
| - | If the host is able to clear the infection, eventually the HBsAg will become undetectable and will be followed by antibodies to the hepatitis B surface antigen (anti-HBs). |
A person negative for HBsAg but positive for anti-HBs has either cleared an infection or has been vaccinated previously. A number of persons who are positive for HBsAg may have very little viral multiplication, and hence may be at little risk of long-term complications or of transmitting infection to others.
More recently, PCR tests have been developed to detect and measure the amount of viral nucleic acid in clinical specimens. These tests are useful to assess a person's infection status and to monitor treatment.

Diagnostic Criteria
| Stage of Hepatitis B | Lab Findings |
|---|---|
| Chronic State | 1. HBsAg-positive >6 months |
| 2. Serum HBV DNA >20,000 IU/mL (105copies/mL), lower values 2,000-
20,000 IU/mL (104-105copies/mL) are often seen in HBeAg-negative chronic hepatitis B | |
| 3. Persistent or intermittent elevation in ALT/AST levels | |
| 4. Liver biopsy showing chronic hepatitis with moderate or severe necroinflammation | |
| Carrier State | 1. HBsAg-positive >6 months |
| 2. HBeAg–, anti-HBe+ | |
| 3. Serum HBV DNA <2,000 IU/mL | |
| 4. Persistently normal ALT/AST levels | |
| 5. Liver biopsy confirms absence of significant hepatitis | |
| Resolved | 1. Previous known history of acute or chronic hepatitis B or the presence of anti-HBc ± anti-HBs |
| 2. HBsAg- | |
| 3. Undetectable serum HBV DNA | |
| 4. Normal ALT levels |
Recommendations for Monitoring Patients with Chronic HBV Infection: AASLD Practice Guidelines 2009[8]
| “ |
1. HBeAg-positive and HBeAg-negative patients who meet criteria for chronic hepatitis B should be evaluated for treatment. (Grade I) 2. HBeAg-positive patients:
3. HBeAg-negative patients:
|
” |
References
- ↑ Bonino F, Chiaberge E, Maran E, Piantino P (1987). "Serological markers of HBV infectivity". Ann. Ist. Super. Sanita. 24 (2): 217–23. PMID 3331068.
- ↑ Lok AS, McMahon BJ (2007). "Chronic hepatitis B". Hepatology. 45 (2): 507–39. doi:10.1002/hep.21513. PMID 17256718.
- ↑ Chu CM, Liaw YF (2007). "Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B". Gastroenterology. 133 (5): 1458–65. doi:10.1053/j.gastro.2007.08.039. PMID 17935720.
- ↑ Zoulim F (2006). "New nucleic acid diagnostic tests in viral hepatitis". Semin. Liver Dis. 26 (4): 309–17. doi:10.1055/s-2006-951602. PMID 17051445.
- ↑ HBsAg at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ HBcAg at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ HBeAg at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Lok AS, McMahon BJ (2004). "[AASLD Practice Guidelines. Chronic hepatitis B: update of therapeutic guidelines]" (PDF). Romanian Journal of Gastroenterology. 13 (2): 150–4. PMID 15229781. Retrieved 2012-02-10. Unknown parameter
|month=ignored (help)