Sandbox:Mehrian: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
|||
| Line 1: | Line 1: | ||
==Pathogenesis== | ==Pathogenesis== | ||
* This form of congenital adrenal hyperplasia results from deficiency of the [[enzyme]] [[17α-hydroxylase]] (also called [[CYP17A1]]). | |||
* This form of congenital adrenal hyperplasia results from deficiency of the [[enzyme]] [[17α-hydroxylase]] (also called [[CYP17A1]]). | |||
* 17α-hydroxylase deficiency impairs the efficiency of [[cortisol]] synthesis, resulting in high levels of [[adrenocorticotropic hormone]] secretion and hyperplasia of the adrenal glands. | * 17α-hydroxylase deficiency impairs the efficiency of [[cortisol]] synthesis, resulting in high levels of [[adrenocorticotropic hormone]] secretion and hyperplasia of the adrenal glands. | ||
* Clinical effects of this condition include overproduction of [[mineralocorticoid]]s and deficiency of prenatal and [[puberty|pubertal]] [[sex steroid]]s. CYP17A1 functions in [[steroidogenesis]], where it converts [[pregnenolone]] and [[progesterone]] to their 17-hydroxy forms. The enzyme itself is attached to the smooth [[endoplasmic reticulum]] of the steroid-producing cells of the [[adrenal gland|adrenal cortex]] and [[gonad]]s | * Clinical effects of this condition include overproduction of [[mineralocorticoid]]s and deficiency of prenatal and [[puberty|pubertal]] [[sex steroid]]s. CYP17A1 functions in [[steroidogenesis]], where it converts [[pregnenolone]] and [[progesterone]] to their 17-hydroxy forms. The enzyme itself is attached to the smooth [[endoplasmic reticulum]] of the steroid-producing cells of the [[adrenal gland|adrenal cortex]] and [[gonad]]s. | ||
===Mineralocorticoid Effects=== | ===Mineralocorticoid Effects=== | ||
* The adrenal cortex is hyperplastic and overstimulated, with no impairment of the mineralocorticoid pathway. Consequently, levels of deoxycorticosterone, [[corticosterone]], and 18-deoxycorticosterone are elevated. Although these precursors of [[aldosterone]] are weaker mineralocorticoids, the extreme elevations usually provide enough volume expansion, blood pressure elevation, and potassium depletion to suppress [[renin]] and aldosterone production. Some persons with 17α-hydroxylase deficiency develop [[hypertension]] in infancy, and nearly 90% do so by late childhood. The low-renin [[hypertension]] is often accompanied by [[hypokalemia]] due to urinary potassium wasting and [[metabolic alkalosis]]. These features of mineralocorticoid excess are the major clinical clue distinguishing the more complete 17α-hydroxylase deficiency from the 17,20-lyase deficiency, which only affects the sex steroids. Treatment with glucocorticoid suppresses ACTH, returns mineralocorticoid production toward normal, and lowers blood pressure. | * The adrenal cortex is hyperplastic and overstimulated, with no impairment of the mineralocorticoid pathway. Consequently, levels of deoxycorticosterone, [[corticosterone]], and 18-deoxycorticosterone are elevated. Although these precursors of [[aldosterone]] are weaker mineralocorticoids, the extreme elevations usually provide enough volume expansion, blood pressure elevation, and potassium depletion to suppress [[renin]] and aldosterone production. Some persons with 17α-hydroxylase deficiency develop [[hypertension]] in infancy, and nearly 90% do so by late childhood. The low-renin [[hypertension]] is often accompanied by [[hypokalemia]] due to urinary potassium wasting and [[metabolic alkalosis]]. These features of mineralocorticoid excess are the major clinical clue distinguishing the more complete 17α-hydroxylase deficiency from the 17,20-lyase deficiency, which only affects the sex steroids. Treatment with glucocorticoid suppresses ACTH, returns mineralocorticoid production toward normal, and lowers blood pressure. | ||
| Line 18: | Line 12: | ||
* A few milder forms of this deficiency in genetic females have allowed relatively normal breast development and irregular menstruation. Evidence suggests that only 5% of normal enzyme activity may be enough to allow at least the physical changes of female puberty, if not [[ovulation]] and fertility. In these girls, the elevated blood pressure was the primary clinical problem. | * A few milder forms of this deficiency in genetic females have allowed relatively normal breast development and irregular menstruation. Evidence suggests that only 5% of normal enzyme activity may be enough to allow at least the physical changes of female puberty, if not [[ovulation]] and fertility. In these girls, the elevated blood pressure was the primary clinical problem. | ||
* 17α-Hydroxylase deficiency in genetic males (XY) results in moderate to severe reduction of fetal [[testosterone]] production by both adrenals and testes. [[virilization|Undervirilization]] is variable and sometimes complete. The appearance of the external [[genitalia]] ranges from normal female to ambiguous to mildly underdeveloped male. The most commonly described phenotype is a small [[phallus]], [[perineum|perineal]] [[hypospadias]], small blind pseudovaginal pouch, and intra-abdominal or [[inguinal canal|inguinal]] testes. [[Wolffian duct]] derivatives are hypoplastic or normal, depending on the degree of testosterone deficiency. Some of those with partial virilization develop [[gynecomastia]] at puberty even though masculinization is reduced. The presence of hypertension in the majority distinguishes them from other forms of partial androgen deficiency or [[androgen insensitivity syndrome|insensitivity]]. Fertility is impaired in those with more than minimal testosterone deficiency. | * 17α-Hydroxylase deficiency in genetic males (XY) results in moderate to severe reduction of fetal [[testosterone]] production by both adrenals and testes. [[virilization|Undervirilization]] is variable and sometimes complete. The appearance of the external [[genitalia]] ranges from normal female to ambiguous to mildly underdeveloped male. The most commonly described phenotype is a small [[phallus]], [[perineum|perineal]] [[hypospadias]], small blind pseudovaginal pouch, and intra-abdominal or [[inguinal canal|inguinal]] testes. [[Wolffian duct]] derivatives are hypoplastic or normal, depending on the degree of testosterone deficiency. Some of those with partial virilization develop [[gynecomastia]] at puberty even though masculinization is reduced. The presence of hypertension in the majority distinguishes them from other forms of partial androgen deficiency or [[androgen insensitivity syndrome|insensitivity]]. Fertility is impaired in those with more than minimal testosterone deficiency. | ||
<references /> | |||
Revision as of 20:11, 3 August 2017
Pathogenesis
- This form of congenital adrenal hyperplasia results from deficiency of the enzyme 17α-hydroxylase (also called CYP17A1).
- 17α-hydroxylase deficiency impairs the efficiency of cortisol synthesis, resulting in high levels of adrenocorticotropic hormone secretion and hyperplasia of the adrenal glands.
- Clinical effects of this condition include overproduction of mineralocorticoids and deficiency of prenatal and pubertal sex steroids. CYP17A1 functions in steroidogenesis, where it converts pregnenolone and progesterone to their 17-hydroxy forms. The enzyme itself is attached to the smooth endoplasmic reticulum of the steroid-producing cells of the adrenal cortex and gonads.
Mineralocorticoid Effects
- The adrenal cortex is hyperplastic and overstimulated, with no impairment of the mineralocorticoid pathway. Consequently, levels of deoxycorticosterone, corticosterone, and 18-deoxycorticosterone are elevated. Although these precursors of aldosterone are weaker mineralocorticoids, the extreme elevations usually provide enough volume expansion, blood pressure elevation, and potassium depletion to suppress renin and aldosterone production. Some persons with 17α-hydroxylase deficiency develop hypertension in infancy, and nearly 90% do so by late childhood. The low-renin hypertension is often accompanied by hypokalemia due to urinary potassium wasting and metabolic alkalosis. These features of mineralocorticoid excess are the major clinical clue distinguishing the more complete 17α-hydroxylase deficiency from the 17,20-lyase deficiency, which only affects the sex steroids. Treatment with glucocorticoid suppresses ACTH, returns mineralocorticoid production toward normal, and lowers blood pressure.
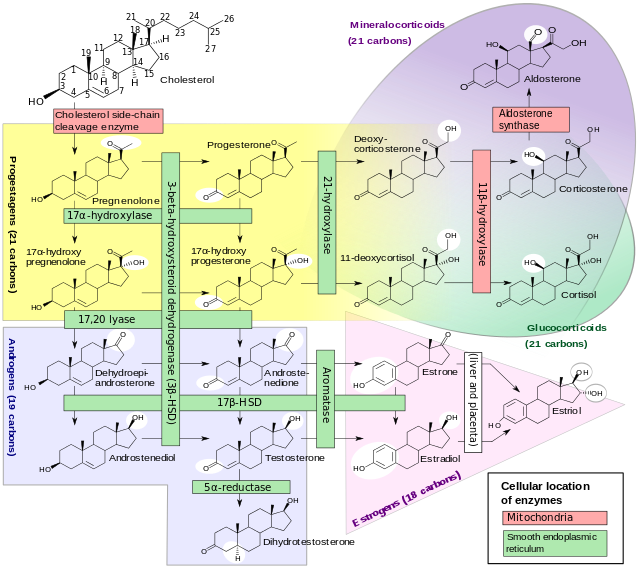
Glucocorticoid Effects
- Although production of cortisol is inefficient enough to normalize ACTH, the 50-100-fold elevations of corticosterone have enough weak glucocorticoid activity to prevent glucocorticoid deficiency and adrenal crisis.
Sex Steroid Effects
- Genetic XX females affected by 17α-hydroxylase deficiency are born with normal female internal and external anatomy. At the expected time of puberty, neither the adrenals nor the ovaries can produce sex steroids, so neither breast development nor pubic hair appears. Investigation of delayed puberty yields elevated gonadotropins and normal karyotype while imaging confirms the presence of ovaries and an infantile uterus. Discovery of hypertension and hypokalemic alkalosis usually suggests the presence of one of the proximal forms of congenital adrenal hyperplasia, and the characteristic mineralocorticoid elevations confirm the specific diagnosis.
- A few milder forms of this deficiency in genetic females have allowed relatively normal breast development and irregular menstruation. Evidence suggests that only 5% of normal enzyme activity may be enough to allow at least the physical changes of female puberty, if not ovulation and fertility. In these girls, the elevated blood pressure was the primary clinical problem.
- 17α-Hydroxylase deficiency in genetic males (XY) results in moderate to severe reduction of fetal testosterone production by both adrenals and testes. Undervirilization is variable and sometimes complete. The appearance of the external genitalia ranges from normal female to ambiguous to mildly underdeveloped male. The most commonly described phenotype is a small phallus, perineal hypospadias, small blind pseudovaginal pouch, and intra-abdominal or inguinal testes. Wolffian duct derivatives are hypoplastic or normal, depending on the degree of testosterone deficiency. Some of those with partial virilization develop gynecomastia at puberty even though masculinization is reduced. The presence of hypertension in the majority distinguishes them from other forms of partial androgen deficiency or insensitivity. Fertility is impaired in those with more than minimal testosterone deficiency.