Von Willebrand disease pathophysiology: Difference between revisions
Nazia Fuad (talk | contribs) No edit summary |
Nazia Fuad (talk | contribs) |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
==Overview== | ==Overview== | ||
Von Willebrand factor is a [[glycoprotein]] present in blood and is involved in [[hemostasis]]. Its [[synthesis]] takes place in the [[endothelium]] (in the Weibel-Palade bodies), [[Megakaryocyte|megakaryocytes]] (α-granules of [[platelets]]), and subendothelial connective tissue and are stored there too. The vWF [[monomer]] contains a number of specific [[Protein domains|domains]] which binds to [[factor VIII]], platelet GPIb-receptor, [[Heparin]], [[Collagen]]. Von Willebrand disease is due to an abnormality, either [[quantitative]] or [[qualitative]], of the von Willebrand factor. Von Willebrand factor gene [[Mutation|mutations]] results in problems with subunit or multimer formation, storage, secretion, [[proteolysis]], and increased clearance. Von Willebrand's Disease can also be [[Acquired disorder|acquired]] secondary to another diseases. [[Acquired disorder|Acquired]] [[VWD]] is associated with other diseases resulting from different pathological processes. These pathological processes includes [[Antibody]] formation resulting in Impaired vWF function and Increased clearance of [[VWF]]. Other mechanisms are enhanced [[proteolysis]] and decreased [[synthesis]] of von Willebrand factor (vWF). [[Von Willebrand disease]] types 1 and 2 (except type 2N which is inherited recessively) are inherited as [[autosomal dominant]] traits and type 3 is inherited as [[autosomal recessive]]. | |||
==Pathophysiology== | ==Pathophysiology== | ||
| Line 15: | Line 16: | ||
'''Structure''' | '''Structure''' | ||
* It is a [[glycoprotein]] present in blood and is involved in [[hemostasis]]. | * It is a [[glycoprotein]] present in blood and is involved in [[hemostasis]]. | ||
* Its [[synthesis]] takes place in the [[endothelium]] (in the Weibel-Palade bodies), [[Megakaryocyte|megakaryocytes]] (α-granules of platelets), and subendothelial connective tissue. | * Its [[synthesis]] takes place in the [[endothelium]] (in the Weibel-Palade bodies), [[Megakaryocyte|megakaryocytes]] (α-granules of [[platelets]]), and [[subendothelial]] [[connective tissue]]. | ||
* The fundamental vWF [[monomer]] is a 2050-[[Amino acid|amino acid protein]]. | * The fundamental vWF [[monomer]] is a 2050-[[Amino acid|amino acid protein]]. | ||
These [[monomer]] contains a number of specific [[Protein domains|domains]] with a distinguishing function. | These [[monomer]] contains a number of specific [[Protein domains|domains]] with a distinguishing function. | ||
| Line 40: | Line 41: | ||
* Von Willebrand factor protects [[Factor VIII|FVIII]] from [[degradation]] and delivers it to the site of injury | * Von Willebrand factor protects [[Factor VIII|FVIII]] from [[degradation]] and delivers it to the site of injury | ||
* [[Factor VIII]] degrades rapidly when not bound to vWF | * [[Factor VIII]] degrades rapidly when not bound to vWF | ||
* [[Factor VIII]] is released from vWF by the action of thrombin. | * [[Factor VIII]] is released from vWF by the action of [[thrombin]]. | ||
* In the absence of vWF, [[factor VIII]] has a [[half-life]] of 1-2 hours | * In the absence of vWF, [[factor VIII]] has a [[half-life]] of 1-2 hours | ||
* When it is carried by intact vWF, [[factor VIII]] [[half-life]] becomes 8-12 hour | * When it is carried by intact vWF, [[factor VIII]] [[half-life]] becomes 8-12 hour | ||
* When vWF is exposed in [[endothelial cells]] due to damage occurring to the blood vessel, it binds to [[collagen]]. | * When vWF is exposed in [[endothelial cells]] due to damage occurring to the blood vessel, it binds to [[collagen]]. | ||
* [[Endothelium]] also releases vWF which forms additional links between the [[Glycoprotein Ib|platelets' glycoprotein Ib]]/IX/V and the collagen fibrils | * [[Endothelium]] also releases vWF which forms additional links between the [[Glycoprotein Ib|platelets' glycoprotein Ib]]/IX/V and the [[Collagen|collagen fibrils]] | ||
* Von Willebrand factor attaches to [[Platelet|platelets]] by its specific receptor to [[glycoprotein Ib]] on the [[platelet]] surface. | * Von Willebrand factor attaches to [[Platelet|platelets]] by its specific receptor to [[glycoprotein Ib]] on the [[platelet]] surface. | ||
* It acts as an adhesive bridge between the [[Platelet|platelets]] and damaged [[subendothelium]] at the site o[[Vascular|f vascular]] injury | * It acts as an adhesive bridge between the [[Platelet|platelets]] and damaged [[subendothelium]] at the site o[[Vascular|f vascular]] injury | ||
| Line 143: | Line 144: | ||
====Heart-related conditions==== | ====Heart-related conditions==== | ||
*[[Mitral valve prolapse]] | *[[Mitral valve prolapse]] | ||
*Ventricular assist device | *Ventricular assist device | ||
*[[Ventricular septal defect]] | *[[Ventricular septal defect]] | ||
*[[Aortic stenosis]] | *[[Aortic stenosis]] | ||
====Malignant diseases==== | ====Malignant diseases==== | ||
*[[Monoclonal gammopathy]] of undetermined significance | *[[Monoclonal gammopathy]] of undetermined significance | ||
*[[chronic myeloid leukemia]] and [[chronic lymphocytic leukemia]] | |||
* | |||
*[[Wilms tumor]] | *[[Wilms tumor]] | ||
*[[Waldenström macroglobulinemia]] | *[[Waldenström macroglobulinemia]] | ||
Latest revision as of 14:10, 5 October 2018
|
Von Willebrand disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Von Willebrand disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Von Willebrand disease pathophysiology |
|
Risk calculators and risk factors for Von Willebrand disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Prince Tano Djan, BSc, MBChB [2] Nazia Fuad M.D.
Overview
Von Willebrand factor is a glycoprotein present in blood and is involved in hemostasis. Its synthesis takes place in the endothelium (in the Weibel-Palade bodies), megakaryocytes (α-granules of platelets), and subendothelial connective tissue and are stored there too. The vWF monomer contains a number of specific domains which binds to factor VIII, platelet GPIb-receptor, Heparin, Collagen. Von Willebrand disease is due to an abnormality, either quantitative or qualitative, of the von Willebrand factor. Von Willebrand factor gene mutations results in problems with subunit or multimer formation, storage, secretion, proteolysis, and increased clearance. Von Willebrand's Disease can also be acquired secondary to another diseases. Acquired VWD is associated with other diseases resulting from different pathological processes. These pathological processes includes Antibody formation resulting in Impaired vWF function and Increased clearance of VWF. Other mechanisms are enhanced proteolysis and decreased synthesis of von Willebrand factor (vWF). Von Willebrand disease types 1 and 2 (except type 2N which is inherited recessively) are inherited as autosomal dominant traits and type 3 is inherited as autosomal recessive.
Pathophysiology
Physiology
Von Willebrand factor (vWF)
The normal physiology of von Willebrand’s factor can be understood as follows:[1]
Structure
- It is a glycoprotein present in blood and is involved in hemostasis.
- Its synthesis takes place in the endothelium (in the Weibel-Palade bodies), megakaryocytes (α-granules of platelets), and subendothelial connective tissue.
- The fundamental vWF monomer is a 2050-amino acid protein.
These monomer contains a number of specific domains with a distinguishing function.
- D'/D3 domain, binds to factor VIII
- A1 domain, which binds to:
- the A2 domain unfolds and then attach to ADAMTS13 protease that inactivates vWF by making smaller multimers.
- The partial unfolding is affected by shear flow in the blood, by calcium binding, and by the "vicinal disulfide" at the A2-domain C-terminus.
- the A3 domain binds to collagen (Von Willebrand factor type A domain)
- the C1 domain, in which the RGD motif binds to platelet integrin αIIbβ3 when this is activated (Von Willebrand factor type C domain)
- Platelet integrin are the transmembrane receptors which facilitate extracellular matrix adhesion.
- The "cystine knot" domain (at the C-terminal end of the protein), which vWF shares with platelet-derived growth factor(PDGF), transforming growth factor-β (TGFβ) and β-human chorionic gonadotropin (βHCG, of pregnancy test fame). (Von Willebrand factor type C domain)
- Monomers are subsequently N-glycosylated, arranged into dimers in the endoplasmic reticulum and into multimers in the Golgi apparatus.
- Multimers of vWF are usually very large, >20,000 kDa, and consist of almost 80 subunits.
- Only the large multimers are functional.
- Some cleavage products from vWF production that are also secreted but serve no function.
Function
- Von Willebrand factor's main function is binding to other proteins, specially factor VIII.
- It plays an important role in platelet adhesion to wound sites.
- Von Willebrand factor binds to factor VIII while it is inactive in circulation
- Von Willebrand factor protects FVIII from degradation and delivers it to the site of injury
- Factor VIII degrades rapidly when not bound to vWF
- Factor VIII is released from vWF by the action of thrombin.
- In the absence of vWF, factor VIII has a half-life of 1-2 hours
- When it is carried by intact vWF, factor VIII half-life becomes 8-12 hour
- When vWF is exposed in endothelial cells due to damage occurring to the blood vessel, it binds to collagen.
- Endothelium also releases vWF which forms additional links between the platelets' glycoprotein Ib/IX/V and the collagen fibrils
- Von Willebrand factor attaches to platelets by its specific receptor to glycoprotein Ib on the platelet surface.
- It acts as an adhesive bridge between the platelets and damaged subendothelium at the site of vascular injury
- VWF binds to platelet glycoprotein Ib when it forms a complex with gpIX and gpV
- This binding is most efficient under high shear stress
- When coagulation has been stimulated vWF binds to other platelet receptors that are activated by thrombin.
Pathogenesis
von Willebrand disease is due to an abnormality, either quantitative or qualitative, of the von Willebrand factor[2]
Pathogenetic mechanisms of inherited VWD
| VWD subtype | Pathogenetic mechanisms | |
|---|---|---|
| Type 1 VWD | 65% have VWF mutations | Partial quantitative deficiency of VWF |
| 70% of VWF variants are missense substitutions affecting VWF trafficking, storage, secretion, and clearance | ||
| Transcription and splicing VWF mutations | ||
| Type 2A VWD | Mutations in D1/D2/D′D3 assemblies, A2 and CTCK domains | Loss of high-molecular-weight multimers (HMWMs) |
| Interference with HMW multimer formation, storage, and secretion | ||
| Increased ADAMTS13 proteolysis | ||
| Type 2B VWD | Mutations in A1 domain | The increase in binding of larger VWF multimers to platelet GP Ib results in sequestration of the platelets and VWF resulting in Thrombocytopenia |
| Excessive binding to GPIb glycoprotein Ib | ||
| Type 2M VWD | Mutations in A1 and A3 domains | Qualitative variants with decreased binding of VWF to GP Ib, resulting in decreased platelet adhesion |
| Diminished binding to GPIbα glycoprotein Ib (A1 domain) or collagen (A3 domain) | ||
| Type 2N VWD | Missense variants in D′D3 assembly | Qualitative variants with remarkably decreased affinity for FVIII |
| Reduced FVIII binding | ||
| Type 3 VWD | VWF mutations found in 85%-90% of cases | Produces null phenotype or the VWF that is not secreted. |
| VWF deletions, nonsense, splice site, and missense mutations |
Pathogenetic mechanisms of acquired VWD
- Von Willebrand's Disease can also be acquired secondary to another disease.[3][4][5][6][7][8][9][10]
- Acquired VWD is associated with other diseases resulting from different pathological processes. These pathological processes include:
- Antibody formation resulting in:[11][12]
- Impaired vWF function
- Increased clearance of VWF
- Enhanced proteolysis
- Decreased synthesis
Genetics
Von Willebrand disease types 1 and 2 (except type 2N which is inherited recessively) are inherited as autosomal dominant traits and type 3 is inherited as autosomal recessive. The diagram below illustrates autosomal dominant inheritance.
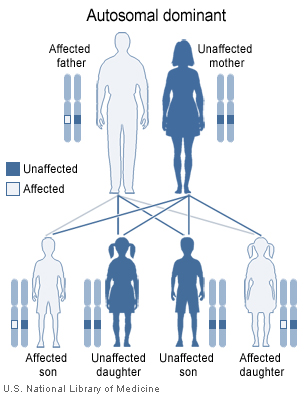 |
- The vWF gene is located on chromosome twelve (12p13.2).
- It has 52 exons spanning 178kbp. *
- VWD Types 1 and 2 are inherited as autosomal dominant traits and type 3 is inherited as autosomal recessive. Occasionally type 2 also inherits recessively.
Associated conditions
Acquired conditions associated with Von Willebrand disease include the following:[13][14][3][4][5][6][7]
- Mitral valve prolapse
- Ventricular assist device
- Ventricular septal defect
- Aortic stenosis
Malignant diseases
- Monoclonal gammopathy of undetermined significance
- chronic myeloid leukemia and chronic lymphocytic leukemia
- Wilms tumor
- Waldenström macroglobulinemia
- Essential thrombocythemia
- Multiple myeloma
- Non-Hodgkin lymphoma
- Polycythemia vera
Drugs and other agents
Autoimmune disorders
Other disorders
References
- ↑ Peyvandi F, Garagiola I, Baronciani L (May 2011). "Role of von Willebrand factor in the haemostasis". Blood Transfus. 9 Suppl 2: s3–8. doi:10.2450/2011.002S. PMC 3159913. PMID 21839029.
- ↑ Lillicrap D (November 2013). "von Willebrand disease: advances in pathogenetic understanding, diagnosis, and therapy". Blood. 122 (23): 3735–40. doi:10.1182/blood-2013-06-498303. PMC 3952678. PMID 24065240.
- ↑ 3.0 3.1 Franchini M, Lippi G (2007). "Acquired von Willebrand syndrome: an update". Am J Hematol. 82 (5): 368–75. doi:10.1002/ajh.20830. PMID 17133419.
- ↑ 4.0 4.1 Tiede A, Rand JH, Budde U, Ganser A, Federici AB (2011). "How I treat the acquired von Willebrand syndrome". Blood. 117 (25): 6777–85. doi:10.1182/blood-2010-11-297580. PMID 21540459.
- ↑ 5.0 5.1 Kumar S, Pruthi RK, Nichols WL (2002). "Acquired von Willebrand disease". Mayo Clin Proc. 77 (2): 181–7. doi:10.4065/77.2.181. PMID 11838652.
- ↑ 6.0 6.1 Veyradier A, Jenkins CS, Fressinaud E, Meyer D (2000). "Acquired von Willebrand syndrome: from pathophysiology to management". Thromb Haemost. 84 (2): 175–82. PMID 10959686.
- ↑ 7.0 7.1 Federici AB, Rand JH, Bucciarelli P, Budde U, van Genderen PJ, Mohri H; et al. (2000). "Acquired von Willebrand syndrome: data from an international registry". Thromb Haemost. 84 (2): 345–9. PMID 10959711.
- ↑ Ng et al. Diagnostic Approach to von Willebrand Disease. Blood 2015; 125(13): 2029-2037.
- ↑ Blomback et al. Von Willebrand Disease Biology Hemophilia 2012; 18: 141-147.
- ↑ Favarolo et al. Von Willebrand Disease and Platelet Disorders. Hemophilia 2014; 20: 59-64.
- ↑ van Genderen PJ, Vink T, Michiels JJ, van 't Veer MB, Sixma JJ, van Vliet HH (1994). "Acquired von Willebrand disease caused by an autoantibody selectively inhibiting the binding of von Willebrand factor to collagen". Blood. 84 (10): 3378–84. PMID 7949092.
- ↑ Handin RI, Martin V, Moloney WC (1976). "Antibody-induced von Willebrand's disease: a newly defined inhibitor syndrome". Blood. 48 (3): 393–405. PMID 1085186.
- ↑ Simone JV, Cornet JA, Abildgaard CF (1968). "Acquired von Willebrand's syndrome in systemic lupus erythematosus". Blood. 31 (6): 806–12. PMID 4172730.
- ↑ Wautier JL, Levy-Toledano S, Caen JP (1976). "Acquired von Willebrand's syndrome and thrombopathy in a patient with chronic lymphocytic leukaemia". Scand J Haematol. 16 (2): 128–34. PMID 1083062.