Dimer
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
|
WikiDoc Resources for Dimer |
|
Articles |
|---|
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Dimer at Clinical Trials.gov Clinical Trials on Dimer at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Dimer
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Dimer Risk calculators and risk factors for Dimer
|
|
Healthcare Provider Resources |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
A dimer is a chemical or biological entity consisting of two subunits called monomers, which are held together by either intramolecular forces (covalent bonds) or weaker intermolecular forces.
Chemistry
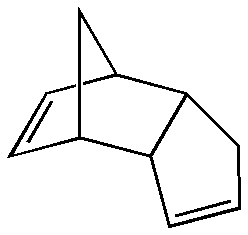
An example of a molecular dimer (i.e. held together by intramolecular forces) is dicyclopentadiene, wherein two cyclopentadiene molecules have reacted to give the product.
Molecular dimers are often formed by the reaction of two identical compounds e.g.: 2A → A-A.
In this example, monomer "A" is said to dimerise to give the dimer "A-A". Diaminocarbenes are another example which dimerise, to give tetraaminoethylenes.
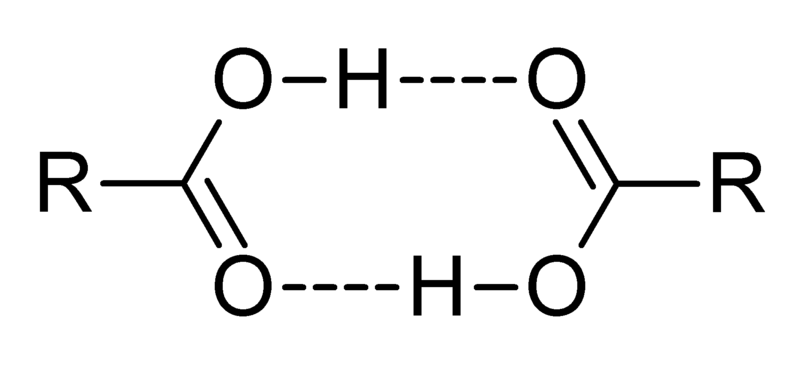
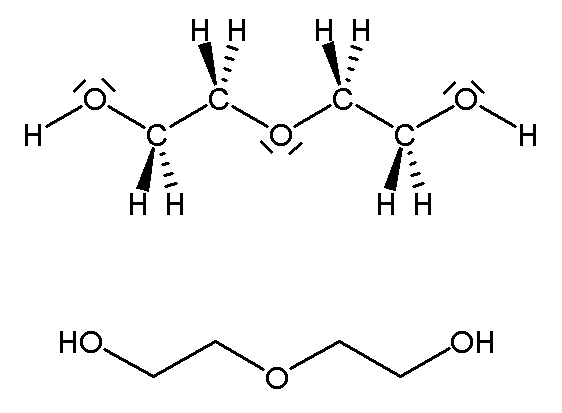
An example of an intermolecular or physical dimer is acetic acid wherein hydrogen bonds hold the two molecules together. The water dimer is another such dimer.
The term homodimer is used when the two molecules are identical (e.g. A-A) and heterodimer when they are not (e.g. A-B).
The reverse of dimerisation is often called disassociation.
Biochemistry
In biochemistry and molecular biology, dimers of macromolecules like proteins and nucleic acids are often observed. The dimerization of identical subunits is called homodimerization; the dimerization of different subunits or unrelated monomers is called heterodimerization. Most dimers in biochemistry are not connected by covalent bonds with the exception of disulfide bridges. An example of this would be the enzyme reverse transcriptase, which is made of two different amino acid chains.
Examples
- Nucleic acids:
- DNA Polymerase
- Proteins:
See also
External links
ar:ثنائي الوحدات de:Dimer ko:이합체 id:Dimer it:Dimero he:דימר nl:Dimeer oc:Dimèr ta:இருபடிச் சேர்மம்