Tobramycin description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Description
TOBI® is a tobramycin solution for inhalation. It is a sterile, clear, slightly yellow, non-pyrogenic, aqueous solution with the pH and salinity adjusted specifically for administration by a compressed air driven reusable nebulizer. The chemical formula for tobramycin is C18H37N5O9 and the molecular weight is 467.52. Tobramycin is O-3-amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribo-hexopyranosyl-(1→6)]-2-deoxy-L-streptamine. The structural formula for tobramycin is:
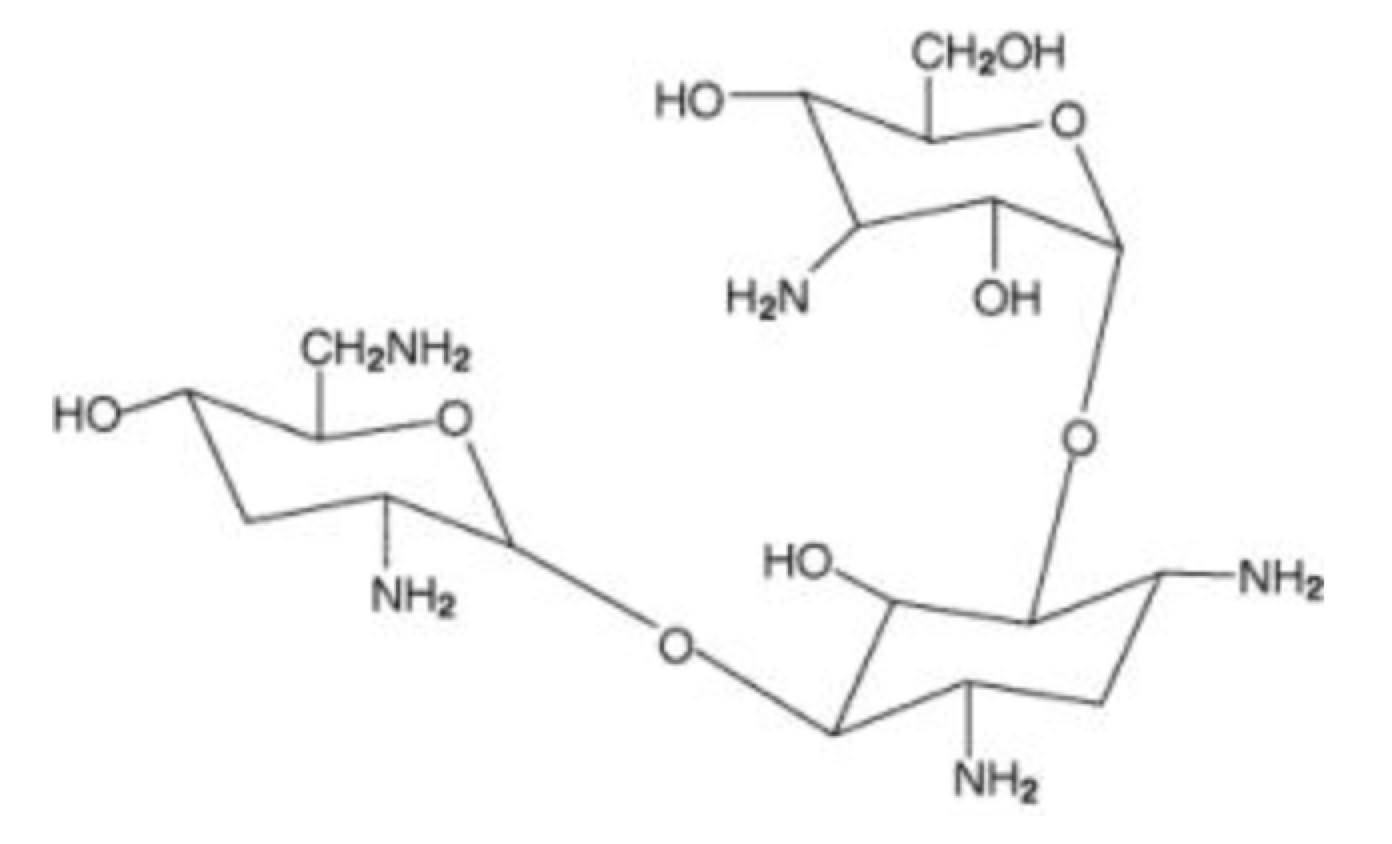 |
Each single-use 5 mL ampule contains 300 mg tobramycin and 11.25 mg sodium chloride in sterile water for injection. Sulfuric acid and sodium hydroxide are added to adjust the pH to 6.0. Nitrogen is used for sparging. All ingredients meet USP requirements. The formulation contains no preservatives.[1]
References
Adapted from the FDA Package Insert.