Test1235
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: DIMINISHED EFFECTIVENESS IN POOR METABOLIZERS
See full prescribing information for complete Boxed Warning.
The effectiveness of clopidogrel bisulfate is dependent on its activation to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. Clopidogrel bisulfate at recommended doses forms less of that metabolite and has a smaller effect on platelet function in patients who are CYP2C19 poor metabolizers. Poor metabolizers with acute coronary syndrome or undergoing percutaneous coronary intervention treated with clopidogrel bisulfate at recommended doses exhibit higher cardiovascular event rates than do patients with normal CYP2C19 function. Tests are available to identify a patient's CYP2C19 genotype; these tests can be used as an aid in determining therapeutic strategy. Consider alternative treatment or treatment strategies in patients identified as CYP2C19 poor metabolizers
|
Overview
Test1235 is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of {{{indication}}}. There is a Black Box Warning for this drug as shown here. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute Coronary Syndrome
- Dosing Information
- For patients with non-ST-elevation ACS (UA/NSTEMI), initiate clopidogrel bisulfate with a single 300 mg oral loading dose and then continue at 75 mg once daily. Initiate aspirin (75 to 325 mg once daily) and continue in combination with clopidogrel bisulfate.
- For patients with STEMI, the recommended dose of clopidogrel bisulfate is 75 mg once daily orally, administered in combination with aspirin (75 to 325 mg once daily), with or without thrombolytics. Clopidogrel bisulfate may be initiated with or without a loading dose.
Recent MI, Recent Stroke, or Established Peripheral Arterial Disease
- Dosing Information
- 75 mg PO qd, with or without food
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Acute ST segment elevation myocardial infarction - Percutaneous coronary intervention - Thrombosis; Prophylaxis
- Dosing Information
- 100 mg
Arterial thrombosis; Prophylaxis
- Dosing Information
- 200 mg
Atrial fibrillation - Thrombosis; Prophylaxis
Heart failure, chronic - Thrombosis; Prophylaxis
Percutaneous coronary intervention, Elective - Thrombosis; Prophylaxis
Non–Guideline-Supported Use
Thrombosis, In patients with stable cardiovascular disease or multiple cardiovascular risk factors; Prophylaxis
- Dosing Information
- 75 mg PO qd
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Test1235 FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Test1235 in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Test1235 in pediatric patients.
Contraindications
- Active Bleeding
- Clopidogrel tablets USP are contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage.
- Hypersensitivity
- Clopidogrel tablets USP are contraindicated in patients with hypersensitivity (e.g., anaphylaxis) to clopidogrel or any component of the product.
Warnings
|
WARNING: DIMINISHED EFFECTIVENESS IN POOR METABOLIZERS
See full prescribing information for complete Boxed Warning.
The effectiveness of clopidogrel bisulfate is dependent on its activation to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. Clopidogrel bisulfate at recommended doses forms less of that metabolite and has a smaller effect on platelet function in patients who are CYP2C19 poor metabolizers. Poor metabolizers with acute coronary syndrome or undergoing percutaneous coronary intervention treated with clopidogrel bisulfate at recommended doses exhibit higher cardiovascular event rates than do patients with normal CYP2C19 function. Tests are available to identify a patient's CYP2C19 genotype; these tests can be used as an aid in determining therapeutic strategy. Consider alternative treatment or treatment strategies in patients identified as CYP2C19 poor metabolizers
|
Diminished Antiplatelet Activity Due to Impaired CYP2C19 Function
Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is achieved through an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by genetic variations in CYP2C19 and by concomitant medications that interfere with CYP2C19.
Proton Pump Inhibitors
Avoid concomitant use of clopidogrel bisulfate with omeprazole or esomeprazole because both significantly reduce the antiplatelet activity of clopidogrel bisulfate.
General Risk of Bleeding
Thienopyridines, including clopidogrel bisulfate, increase the risk of bleeding. If a patient is to undergo surgery and an antiplatelet effect is not desired, discontinue clopidogrel bisulfate five days prior to surgery. In patients who stopped therapy more than five days prior to CABG the rates of major bleeding were similar (event rate 4.4% clopidogrel bisulfate + aspirin; 5.3% placebo + aspirin). In patients who remained on therapy within five days of CABG, the major bleeding rate was 9.6% for clopidogrel bisulfate + aspirin, and 6.3% for placebo + aspirin. Thienopyridines inhibit platelet aggregation for the lifetime of the platelet (7 to 10 days), so withholding a dose will not be useful in managing a bleeding event or the risk of bleeding associated with an invasive procedure. Because the half-life of clopidogrel's active metabolite is short, it may be possible to restore hemostasis by administering exogenous platelets; however, platelet transfusions within 4 hours of the loading dose or 2 hours of the maintenance dose may be less effective.
Discontinuation of clopidogrel bisulfate
Avoid lapses in therapy, and if clopidogrel bisulfate must be temporarily discontinued, restart as soon as possible. Premature discontinuation of clopidogrel bisulfate may increase the risk of cardiovascular events.
Patients with Recent Transient Ischemic Attack (TIA) or Stroke
In patients with recent TIA or stroke who are at high risk for recurrent ischemic events, the combination of aspirin and clopidogrel bisulfate has not been shown to be more effective than clopidogrel bisulfate alone, but the combination has been shown to increase major bleeding.
Thrombotic Thrombocytopenic Purpura (TTP)
TTP, sometimes fatal, has been reported following use of clopidogrel bisulfate, sometimes after a short exposure (<2 weeks). TTP is a serious condition that requires urgent treatment including plasmapheresis (plasma exchange). It is characterized by thrombocytopenia, microangiopathic hemolytic anemia (schistocytes [fragmented RBCs] seen on peripheral smear), neurological findings, renal dysfunction, and fever.
Cross-Reactivity among Thienopyridines
Hypersensitivity including rash, angioedema or hematologic reaction have been reported in patients receiving clopidogrel bisulfate, including patients with a history of hypersensitivity or hematologic reaction to other thienopyridines.
Adverse Reactions
Clinical Trials Experience
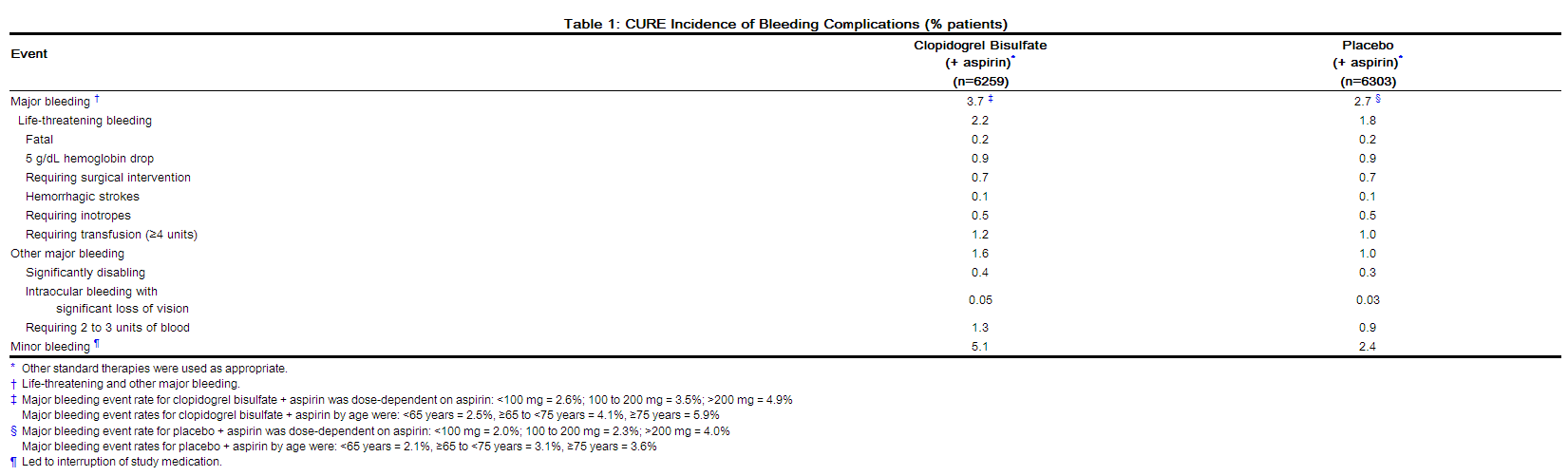
The following serious adverse reactions are discussed below and elsewhere in the labeling: Bleeding [see Warnings and Precautions (5.2)] Thrombotic thrombocytopenic purpura [see Warnings and Precautions (5.5)] 6.1 Clinical Studies Experience Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Clopidogrel bisulfate has been evaluated for safety in more than 54,000 patients, including over 21,000 patients treated for 1 year or more. The clinically important adverse reactions observed in trials comparing clopidogrel bisulfate plus aspirin to placebo plus aspirin and trials comparing clopidogrel bisulfate alone to aspirin alone are discussed below. Bleeding CURE In CURE, clopidogrel bisulfate use with aspirin was associated with an increase in major bleeding (primarily gastrointestinal and at puncture sites) compared to placebo with aspirin (see Table 1). The incidence of intracranial hemorrhage (0.1%) and fatal bleeding (0.2%) were the same in both groups. Other bleeding events that were reported more frequently in the clopidogrel group were epistaxis, hematuria, and bruise. The overall incidence of bleeding is described in the table below.
Postmarketing Experience
There is limited information regarding Test1235 Postmarketing Experience in the drug label.
Drug Interactions
= SSRIs and SNRIs
Since selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) affect platelet activation, the concomitant administration of SSRIs and SNRIs with clopidogrel may increase the risk of bleeding.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Reproduction studies performed in rats and rabbits at doses up to 500 and 300 mg/kg/day, respectively (65 and 78 times the recommended daily human dose, respectively, on a mg/m2 basis), revealed no evidence of impaired fertility or fetotoxicity due to clopidogrel. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of a human response, clopidogrel bisulfate should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Test1235 in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Test1235 during labor and delivery.
Nursing Mothers
Studies in rats have shown that clopidogrel and/or its metabolites are excreted in the milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from clopidogrel, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric populations have not been established. Additional information describing a clinical study in which efficacy was not demonstrated in neonates and infants is approved in the package insert for Bristol-Myers Squibb’s clopidogrel tablets. However, due to Bristol-Myers Squibb’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Geriatic Use
There is no FDA guidance on the use of Test1235 in geriatric settings.
Gender
There is no FDA guidance on the use of Test1235 with respect to specific gender populations.
Race
There is no FDA guidance on the use of Test1235 with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Test1235 in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Test1235 in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Test1235 in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Test1235 in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Test1235 Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Test1235 and IV administrations.
Overdosage
There is limited information regarding Test1235 overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
There is limited information regarding Test1235 Mechanism of Action in the drug label.
Structure
There is limited information regarding Test1235 Structure in the drug label.
Pharmacodynamics
There is limited information regarding Test1235 Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Test1235 Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Test1235 Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Test1235 Clinical Studies in the drug label.
How Supplied
There is limited information regarding Test1235 How Supplied in the drug label.
Storage
There is limited information regarding Test1235 Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Test1235 |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Test1235 |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Test1235 Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Test1235 interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- 1
- 2
- 3
Look-Alike Drug Names
Plavix - Paxil
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Test1235
|Pill Name=
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Test1235 |Label Name=
}}