Terconazole
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Terconazole is an antifungal that is FDA approved for the treatment of vulvovaginal candidiasis (moniliasis). Common adverse reactions include vulvovaginal burning, itching, headache, dysmenorrhea, abdominal pain, asthenia, Influenza-Like Illness consisting of multiple listed reactions including fever and chills, nausea, vomiting, myalgia, arthralgia, malaise, hypersensitivity, anaphylaxis, face edema, bronchospasm, rash, toxic epidermal necrolysis, urticaria.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Terconazole Vaginal Cream 0.4%
- One full applicator (5 g) of Terconazole Vaginal Cream (20 mg terconazole) should be administered intravaginally once daily at bedtime for seven consecutive days.
Terconazole Vaginal Cream 0.8%
- One full applicator (5 g) of Terconazole Vaginal Cream (40 mg terconazole) should be administered intravaginally once daily at bedtime for three consecutive days.
- Before prescribing another course of therapy, the diagnosis should be reconfirmed by smears and/or cultures and other pathogens commonly associated with vulvovaginitis ruled out. The therapeutic effect of these products is not affected by menstruation.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Terconazole in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Terconazole in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Terconazole FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Terconazole in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Terconazole in pediatric patients.
Contraindications
Patients known to be hypersensitive to terconazole or to any of the components of the cream.
Warnings
- Anaphylaxis and toxic epidermal necrolysis have been reported during terconazole therapy. Terconazole therapy should be discontinued if anaphylaxis or toxic epidermal necrolysis develops.
Adverse Reactions
Clinical Trials Experience
Adverse Reactions from Clinical Trials
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Terconazole Vaginal Cream 0.4%
- During controlled clinical studies conducted in the United States, 521 patients with vulvovaginal candidiasis were treated with terconazole 0.4% vaginal cream.
- Based on comparative analyses with placebo, the adverse experiences considered most likely related to terconazole 0.4% vaginal cream were headache (26% vs. 17% with placebo) and body pain (2.1% vs. 0% with placebo). Fever (1.7% vs. 0.5% with placebo) and chills (0.4% vs. 0.0% with placebo), vulvovaginal burning, itching and irritation have also been reported.
- The adverse drug experience on terconazole most frequently causing discontinuation was vulvovaginal itching.
Terconazole Vaginal Cream 0.8%
- During controlled clinical studies conducted in the United States, patients with vulvovaginal candidiasis were treated with terconazole 0.8% vaginal cream for three days.
- Based on comparative analyses with placebo and a standard agent, the adverse experiences considered most likely related to terconazole 0.8% vaginal cream were headache (21% vs. 16% with placebo) and dysmenorrhea (6% vs. 2% with placebo).
- Other adverse experiences reported with terconazole 0.8% vaginal cream were abdominal pain (3.4% vs. 1% with placebo) and fever (1% vs. 0.3% with placebo).
- The adverse drug experience most frequently causing discontinuation of therapy was vulvovaginal itching, 0.7% with the terconazole 0.8% vaginal cream group and 0.3% with the placebo group.
Postmarketing Experience
- The following adverse drug reactions have been first identified during post-marketing experience with terconazole.
- Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General
- Asthenia, Influenza-Like Illness consisting of multiple listed reactions including fever and chills, nausea, vomiting, myalgia, arthralgia, malaise
Immune
- Hypersensitivity, Anaphylaxis, Face Edema
Nervous
- Dizziness
Respiratory
- Bronchospasm
Skin
- Rash, Toxic Epidermal Necrolysis, Urticaria
Drug Interactions
There is limited information regarding Terconazole Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Terconazole in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Terconazole in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Terconazole during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Terconazole in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Terconazole in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Terconazole in geriatric settings.
Gender
There is no FDA guidance on the use of Terconazole with respect to specific gender populations.
Race
There is no FDA guidance on the use of Terconazole with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Terconazole in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Terconazole in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Terconazole in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Terconazole in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Terconazole Administration in the drug label.
Monitoring
There is limited information regarding Terconazole Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Terconazole and IV administrations.
Overdosage
- In the rat, the oral LD 50 values were found to be 1741 and 849 mg/kg for the male and female, respectively. The oral LD 50 values for the male and female dog were ≅1280 and ≥640 mg/kg, respectively.
- In the event of oral ingestion of cream, supportive and symptomatic measures should be carried out. If the cream is accidentally applied to the eyes, wash with clean water or saline and seek medical attention if symptoms persist.
Pharmacology
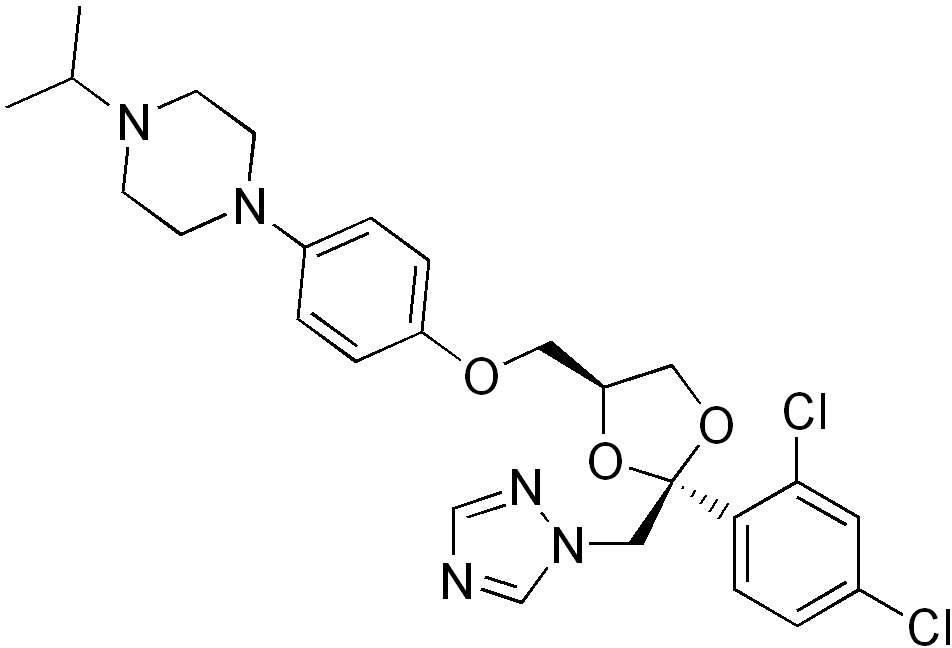
| |
Terconazole
| |
| Systematic (IUPAC) name | |
| 1-[4-[ [(2S,4S)-2-(2,4-Dichlorophenyl)-2- (1,2,4-triazol-1-ylmethyl)- 1,3-dioxolan-4-yl]methoxy]phenyl]- 4-propan-2-yl-piperazine | |
| Identifiers | |
| CAS number | |
| ATC code | G01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 532.462 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 94.9% |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Terconazole, an azole antifungal agent, inhibits fungal cytochrome P-450-mediated 14 alpha-lanosterol demethylase enzyme. This enzyme functions to convert lanosterol to ergosterol.
- The accumulation of 14 alpha-methyl sterols correlates with the subsequent loss of ergosterol in the fungal cell wall and may be responsible for the antifungal activity of terconazole. Mammalian cell demethylation is less sensitive to terconazole inhibition.
Activity in vitro
- Terconazole exhibits antifungal activity in vitro against Candida albicans and other Candida species. The MIC values of terconazole against most Lactobacillus spp. typically found in the human vagina were ≥128 mcg/mL; therefore these beneficial bacteria are not affected by drug treatment.
Structure
There is limited information regarding Terconazole Structure in the drug label.
Pharmacodynamics
There is limited information regarding Terconazole Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- Following a single intravaginal application of a suppository containing 240 mg 14C-terconazole to healthy women, approximately 70% (range: 64–76%) of terconazole remains in the vaginal area during the suppository retention period (16 hours); approximately 10% (range: 5–16%) of the administered radioactivity was absorbed systemically over 7 days.
- Maximum plasma concentrations of terconazole occur 5 to 10 hours after intravaginal application of the cream or suppository. Systemic exposure to terconazole is approximately proportional to the applied dose, whether as the cream or suppository.
- The rate and extent of absorption of terconazole are similar in patients with vulvovaginal candidiasis (pregnant or non-pregnant) and healthy subjects.
Distribution
- Terconazole is highly protein bound (94.9%) in human plasma and the degree of binding is independent of drug concentration over the range of 0.01 to 5.0 mcg/mL.
Metabolism
- Systemically absorbed terconazole is extensively metabolized (>95%).
Elimination
- Across various studies in healthy women, after single or multiple intravaginal administration of terconazole as the cream or suppository/ovule, the mean elimination half-life of unchanged terconazole ranged from 6.4 to 8.5 hours.
- Following a single intravaginal administration of a suppository containing 240 mg 14C-terconazole to hysterectomized or tubal ligated women, approximately 3 to 10% (mean ± SD: 5.7 ± 3.0%) of the administered radioactivity was eliminated in the urine and 2 to 6% (mean ± SD: 4.2 ± 1.6%) was eliminated in the feces during the 7-day collection period.
Multiple Dosing
- There is no significant increase in maximum plasma concentration or overall exposure (AUC) after multiple daily applications of the cream for 7 days or suppositories for 3 days.
- Photosensitivity reactions were observed in some normal volunteers following repeated dermal application of terconazole 2.0% and 0.8% creams under conditions of filtered artificial ultraviolet light.
- Photosensitivity reactions have not been observed in U.S. and foreign clinical trials in patients who were treated with terconazole suppositories or vaginal cream (0.4% and 0.8%).
Nonclinical Toxicology
There is limited information regarding Terconazole Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Terconazole Clinical Studies in the drug label.
How Supplied
- Terconazole Vaginal Cream 0.4% is available in 45 g (NDC 0591-3196-89) tubes with a measured-dose applicator. Store at Controlled Room Temperature 15°–30°C (59°–86°F).
- Terconazole Vaginal Cream 0.8% is available in 20 g (NDC 0591-3197-52) tubes with a measured-dose applicator.
Storage
- Store at Controlled Room Temperature 15°–30°C (59°–86°F).
Images
Drug Images
{{#ask: Page Name::Terconazole |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Terconazole |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- For best results, be sure to use the medication as prescribed by your doctor, even if you feel better quickly.
- Avoid sexual intercourse, if your doctor advises you to do so.
- If your partner has any penile itching, redness, or discomfort, he should consult his physician and mention that you are being treated for a yeast infection.
- You can use the medication even if you are having your menstrual period.
- However, you should not use tampons because they may absorb the medication. * Instead, use external pads or napkins until you have finished your medication. You may also wish to wear a sanitary napkin if the vaginal medication leaks.
- Dry the genital area thoroughly after showering, bathing, or swimming.
- Change out of a wet bathing suit or damp exercise clothes as soon as possible.
- A dry environment is less likely to encourage the growth of yeast.
Wipe from front to rear (away from the vagina) after a bowel movement.
- Don't douche unless your doctor specifically tells you to do so. Douching may disturb the vaginal balance.
- Don't scratch if you can help it. Scratching can cause more irritation and spread the infection.
- Discuss with your physician any medication you are already taking. Certain types of medication can make your vagina more susceptible to infection.
- Eat nutritious meals to promote your general health.
Precautions with Alcohol
Alcohol-Terconazole interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- TERCONAZOLE®[1]
Look-Alike Drug Names
There is limited information regarding Terconazole Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Terconazole |Label Name=Terconazole01.png
}}