Simeprevir drug interactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Drug Interactions
Co-administration of OLYSIO with substances that are moderate or strong inducers or inhibitors of cytochrome P450 3A (CYP3A) is not recommended as this may lead to significantly lower or higher exposure of simeprevir, respectively [see Drug Interactions (7) and Clinical Pharmacology (12.3)]. In vitro studies indicated that simeprevir is a substrate and mild inhibitor of CYP3A and a substrate and inhibitor of P-gp and OATP1B1/3. Simeprevir does not affect CYP2C9, CYP2C19 or CYP2D6 in vivo. Simeprevir does not induce CYP1A2 or CYP3A4 in vitro. In vivo, simeprevir mildly inhibits the CYP1A2 activity and intestinal CYP3A4 activity, while it does not affect hepatic CYP3A4 activity.
Simeprevir is transported into the liver by OATP1B1/3 where it undergoes metabolism by CYP3A. Based on results from in vivo studies, co-administration of OLYSIO with moderate or strong inhibitors of CYP3A may significantly increase the plasma exposure of simeprevir and co-administration with moderate or strong inducers of CYP3A may significantly reduce the plasma exposure of simeprevir, which may lead to loss of efficacy.
Drug interaction studies were performed in healthy adults with simeprevir (at the recommended dose of 150 mg once daily unless otherwise noted) and drugs likely to be co-administered or drugs commonly used as probes for pharmacokinetic interactions. The effects of co-administration of other drugs on the Cmax, AUC, and Cmin values of simeprevir are summarized in Table 6 (effect of other drugs on OLYSIO). The effect of co-administration of OLYSIO on the Cmax, AUC, and Cmin values of other drugs are summarized in Table 7 (effect of OLYSIO on other drugs). For information regarding clinical recommendations, see Drug Interactions (7).
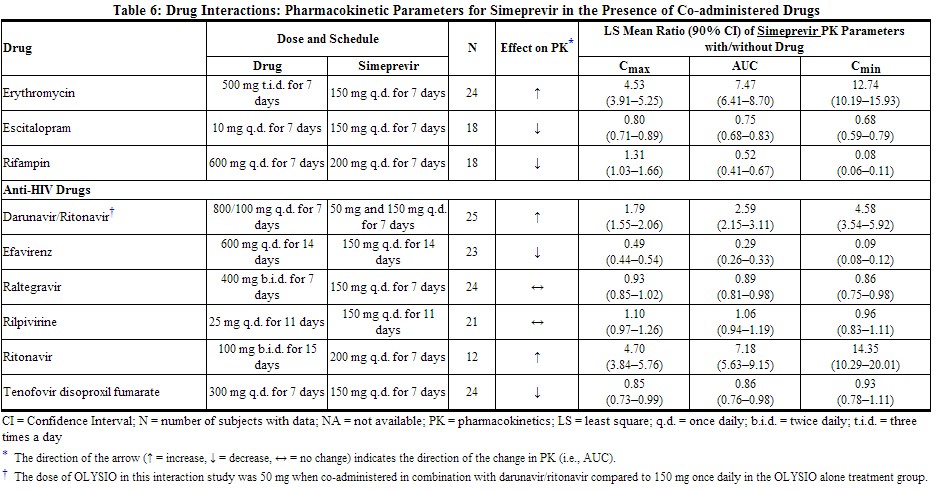 |
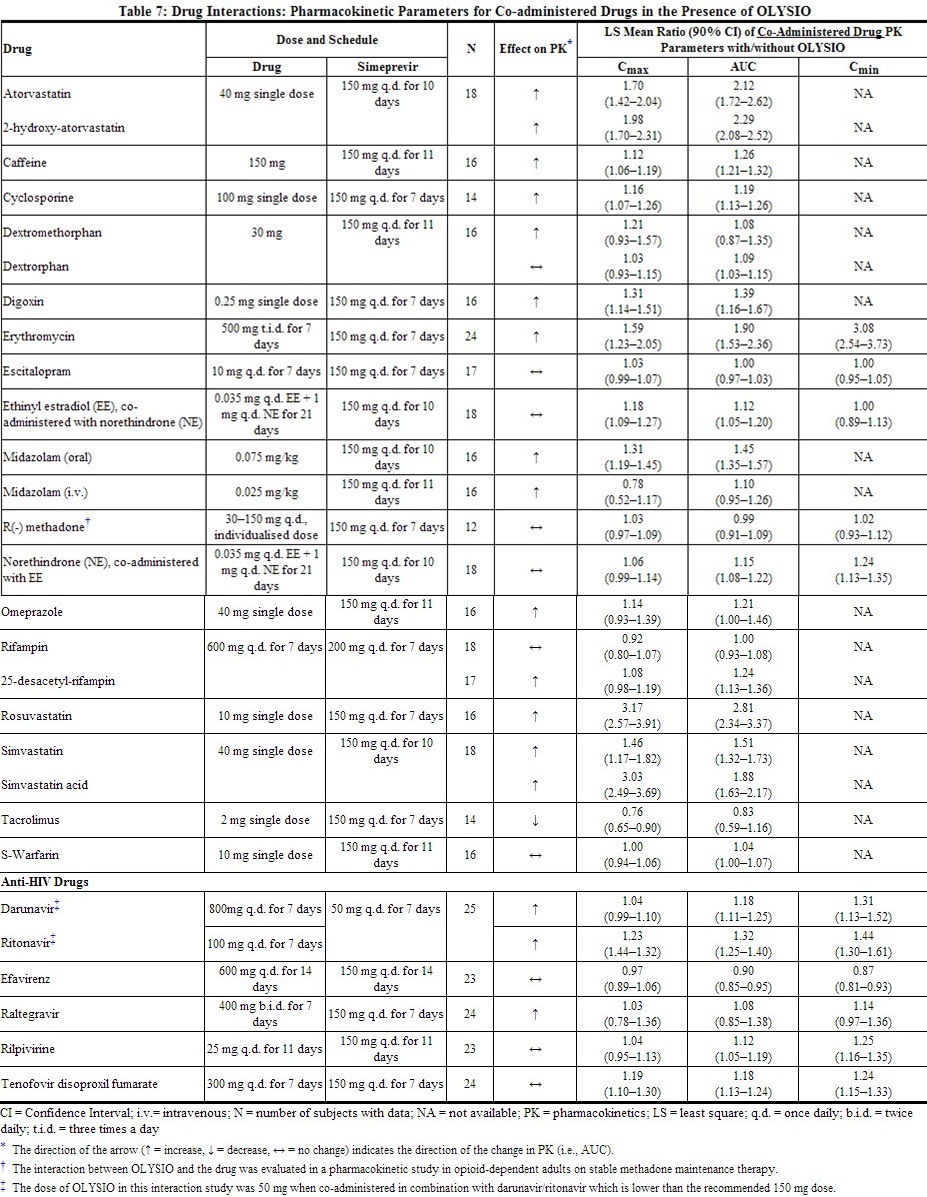 |
References
- ↑ "OLYSIO (SIMEPREVIR) CAPSULE [JANSSEN PRODUCTS LP]". Retrieved 7 January 2014.
Adapted from the FDA Package Insert.