Alirocumab
For a review of all PCSK9 inhibitors please click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tarek Nafee, M.D. [2],Aysha Aslam, M.B.B.S[3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Alirocumab (REGN727 and SAR236553) is an investigational human monoclonal antibody that inhibits PCSK9 for the treatment of hypercholesterolemia. Alirocumab is indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerostic cardiovascular disease, who require additional lowering of LDL-C. The effect of alirocumab on cardiovascular morbidity and mortality has not been determined.
Indication and Dosage
FDA-Labeled Indications and Dosage (Adult)
Heterozygous Familial Hypercholesterolemia
For patients with heterozygous familial hypercholesterolemia
- Initial dose: 75mg/ml SQ q2w
For patients with heterozygous familial hypercholesterolemia and inadequate LDL-C response
- Dose: 150mg/ml SQ q2w
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information about the guideline-supported use.
Non–Guideline-Supported Use
Drug Administration Instructions
- If a dose is missed, instruct the patient to administer the injection within 7 days from the missed dose and then resume the patient's original schedule. If the missed dose is not administered within 7 days, instruct the patient to wait until the next dose on the original schedule.
- Provide proper training to patients and/or caregivers on the preparation and administration of alirocumab prior to use according to the Instructions for Use. Instruct patients and/or caregivers to read and follow the Instructions for Use each time they use alirocumab.
- Allow alirocumab to warm to room temperature for 30 to 40 minutes prior to use. Use alirocumab as soon as possible after it has warmed up. Do NOT use alirocumab if it has been at room temperature [77°F (25°C)] for 24 hours or longer.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. If the solution is discolored or contains visible particulate matter, the solution should not be used.
- Follow aseptic injection technique every time alirocumab is administered.
- Administer alirocumab by subcutaneous injection into the thigh, abdomen, or upper arm using a single-dose pre-filled pen or single-dose pre-filled syringe.
- Rotate the injection site with each injection.
- Do NOT inject alirocumab into areas of active skin disease or injury such as sunburns, skin rashes, inflammation, or skin infections.
- Do NOT co-administer alirocumab with other injectable drugs at the same injection site.
Dosage Forms and Strengths
Alirocumab is a clear, colorless to pale yellow solution available as follows:
Injection: Single-dose pre-filled pen
- 75 mg/mL
- 150 mg/mL
Injection: Single-dose pre-filled syringe
- 75 mg/mL
- 150 mg/mL
Contraindications
Alirocumab is contraindicated in patients with a history of a serious hypersensitivity reaction to alirocumab. Reactions have included hypersensitivity vasculitis and hypersensitivity reactions requiring hospitalization.
Warnings and Precautions
Allergic Reactions
Hypersensitivity reactions (e.g., pruritus, rash, urticaria), including some serious events (e.g., hypersensitivity vasculitis and hypersensitivity reactions requiring hospitalization), have been reported with alirocumab treatment. If signs or symptoms of serious allergic reactions occur, discontinue treatment with alirocumab, treat according to the standard of care, and monitor until signs and symptoms resolve.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of alirocumab was evaluated in 9 placebo-controlled trials that included 2476 patients treated with alirocumab, including 2135 exposed for 6 months and 1999 exposed for more than 1 year (median treatment duration of 65 weeks). The mean age of the population was 59 years, 40% of the population were women, 90% were Caucasians, 4% were Black or African American, and 3% were Asians. At baseline, 37% of patients had a diagnosis of heterozygous familial hypercholesterolemia and 66% had clinical atherosclerotic cardiovascular disease.
- Adverse reactions reported in at least 2% of alirocumab-treated patients, and more frequently than in placebo-treated patients, are shown in the table below:
| Adverse Reactions | Placebo
(N=1276) |
Alirocumab*
(N=2476) |
|---|---|---|
| Nasopharyngitis | 11.1% | 11.3% |
| Injection site reactions† | 5.1% | 7.2% |
| Influenza | 4.6% | 5.7% |
| Urinary tract infection | 4.6% | 4.8% |
| Diarrhea | 4.4% | 4.7% |
| Bronchitis | 3.8% | 4.3% |
| Myalgia | 3.4% | 4.2% |
| Muscle spasms | 2.4% | 3.1% |
| Sinusitis | 2.7% | 3.0% |
| Cough | 2.3% | 2.5% |
| Contusion | 1.3% | 2.1% |
| Musculoskeletal pain | 1.6% | 2.1% |
| ||
Adverse reactions led to discontinuation of treatment in 5.3% of patients treated with alirocumab and 5.1% of patients treated with placebo. The most common adverse reactions leading to treatment discontinuation in patients treated with alirocumab were allergic reactions (0.6% versus 0.2% for alirocumab and placebo, respectively) and elevated liver enzymes (0.3% versus <0.1%).
- Local Injection Site Reactions
Local injection site reactions including erythema/redness, itching, swelling, and pain/tenderness were reported more frequently in patients treated with alirocumab (7.2% versus 5.1% for alirocumab and placebo, respectively). Few patients discontinued treatment because of these reactions (0.2% versus 0.4% for alirocumab and placebo, respectively), but patients receiving alirocumab had a greater number of injection site reactions, had more reports of associated symptoms, and had reactions of longer average duration than patients receiving placebo.
- Allergic Reactions
Allergic reactions were reported more frequently in patients treated with alirocumab than in those treated with placebo (8.6% versus 7.8%). The proportion of patients who discontinued treatment due to allergic reactions was higher among those treated with alirocumab (0.6% versus 0.2% ). Serious allergic reactions, such as hypersensitivity, nummular eczema, and hypersensitivity vasculitis were reported in patients using alirocumab in controlled clinical trials [see Warnings and Precautions (5.1)].
- Neurocognitive Events
Neurocognitive events were reported in 0.8% of patients treated with alirocumab and 0.7% of patients treated with placebo. Confusion or memory impairment were reported more frequently by those treated with alirocumab (0.2% for each) than in those treated with placebo (<0.1% for each).
- Liver Enzyme Abnormalities
Liver-related disorders (primarily related to abnormalities in liver enzymes) were reported in 2.5% of patients treated with alirocumab and 1.8% of patients treated with placebo, leading to treatment discontinuation in 0.4% and 0.2% of patients, respectively. Increases in serum transaminases to greater than 3 times the upper limit of normal occurred in 1.7% of patients treated with alirocumab and 1.4% of patients treated with placebo.
- Low LDL-C Values
In a pool of both placebo- and active-controlled clinical trials, 796 alirocumab-treated patients had two consecutive calculated LDL-C values <25 mg/dL, and 288 had two consecutive calculated LDL-C values <15 mg/dL. Changes to background lipid-altering therapy (e.g., maximally tolerated statins) were not made in response to low LDL-C values, and alirocumab dosing was not modified or interrupted on this basis. Although adverse consequences of very low LDL-C were not identified in these trials, the long-term effects of very low levels of LDL-C induced by alirocumab are unknown.
Immunogenicity
- As with all therapeutic proteins, there is a potential for immunogenicity with alirocumab. In a pool of ten placebo- and active-controlled trials, 4.8% of patients treated with alirocumab had anti-drug antibodies (ADA) newly detected after initiating treatment as compared with 0.6% of patients treated with control.
- Patients who developed ADA had a higher incidence of injection site reactions compared with patients who did not develop ADA (10.2% vs 5.9%).
- A total of 1.2% of patients treated with alirocumab developed neutralizing antibodies (NAb) on at least one occasion as compared with no patients treated with control, and 0.3% of patients both tested positive for NAb and exhibited transient or prolonged loss of efficacy. The long-term consequences of continuing alirocumab treatment in the presence of persistent NAb are unknown.
- Immunogenicity data are highly dependent on the sensitivity and specificity of the assay as well as other factors. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors, including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to alirocumab with the incidence of antibodies to other products may be misleading.
Use in Specific Populations
Pregnancy
Risk Summary
There are no available data on use of alirocumab in pregnant women to inform a drug-associated risk. In animal reproduction studies, there were no effects on embryo-fetal development when rats were subcutaneously administered alirocumab during organogenesis at dose exposures up to 12-fold the exposure at the maximum recommended human dose of 150 mg every two weeks. In monkeys, suppression of the humoral immune response was observed in infant monkeys when alirocumab was dosed during organogenesis to parturition at dose exposures 13-fold the exposure at the maximum recommended human dose of 150 mg every two weeks. No additional effects on pregnancy or neonatal/infant development were observed at dose exposures up to 81-fold the maximum recommended human dose of 150 mg every two weeks. Measurable alirocumab serum concentrations were observed in the infant monkeys at birth at comparable levels to maternal serum, indicating that alirocumab, like other IgG antibodies, crosses the placental barrier. FDA's experience with monoclonal antibodies in humans indicates that they are unlikely to cross the placenta in the first trimester; however, they are likely to cross the placenta in increasing amounts in the second and third trimester. Consider the benefits and risks of alirocumab and possible risks to the fetus before prescribing alirocumab to pregnant women.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data
Animal Data
In Sprague Dawley rats, no effects on embryo-fetal development were observed when alirocumab was dosed at up to 75 mg/kg/dose by the subcutaneous route on gestation days 6 and 12 at exposures 12-fold the maximum recommended human dose of 150 mg every two weeks, based on serum AUC.
In cynomolgus monkeys, suppression of the humoral immune response to keyhole limpet hemocyanin (KLH) antigen was observed in infant monkeys at 4 to 6 months of age when alirocumab was dosed during organogenesis to parturition at 15 mg/kg/week and 75 mg/kg/week by the subcutaneous route, corresponding to 13- and 81-fold the human exposure at the maximum recommended human dose of 150 mg every two weeks, based on serum AUC. The lowest dose tested in the monkey resulted in humoral immune suppression; therefore it is unknown if this effect would be observed at clinical exposure. No study designed to challenge the immune system of infant monkeys was conducted. No additional embryo-fetal, prenatal or postnatal effects were observed in infant monkeys, and no maternal effects were observed, when alirocumab was dosed at up to 75 mg/kg/week by the subcutaneous route, corresponding to maternal exposure of 81-fold the exposure at the maximum recommended human dose of 150 mg every two weeks, based on serum AUC.
Lactation
Risk Summary
There is no information regarding the presence of alirocumab in human milk, the effects on the breastfed infant, or the effects on milk production. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for alirocumab and any potential adverse effects on the breastfed infant from alirocumab or from the underlying maternal condition. Human IgG is present in human milk, but published data suggest that breastmilk IgG antibodies do not enter the neonatal and infant circulation in substantial amounts.
Pediatric Use
Safety and efficacy in pediatric patients have not been established.
Geriatric Use
In controlled studies, 1158 patients treated with alirocumab were ≥65 years of age and 241 patients treated with alirocumab were ≥75 years of age. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Renal Impairment
No dose adjustment is needed for patients with mild or moderately impaired renal function. No data are available in patients with severe renal impairment.
Hepatic Impairment
No dose adjustment is needed for patients with mild or moderate hepatic impairment. No data are available in patients with severe hepatic impairment.
Description
Alirocumab is a human monoclonal antibody (IgG1 isotype) that targets proprotein convertase subtilisin kexin type 9 (PCSK9). Alirocumab is a PCSK9 inhibitor produced by recombinant DNA technology in Chinese Hamster Ovary cell suspension culture. Alirocumab consists of two disulfide-linked human heavy chains, each covalently linked through a disulfide bond to a human kappa light chain. A single N-linked glycosylation site is located in each heavy chain within the CH2 domain of the Fc constant region of the molecule. The variable domains of the heavy and light chains combine to form the PCSK9 binding site within the antibody. Alirocumab has an approximate molecular weight of 146 kDa.
Alirocumab is a sterile, preservative-free, clear, colorless to pale yellow solution for subcutaneous injection. Alirocumab 75 mg/mL or 150 mg/mL solution for subcutaneous injection in a single-dose pre-filled pen or single-dose pre-filled syringe is supplied in a siliconized 1 mL Type-1 clear glass syringe. The needle shield is not made with natural rubber latex.
Each 75 mg/mL pre-filled pen or pre-filled syringe contains 75 mg alirocumab, histidine (8 mM), polysorbate 20 (0.1 mg), sucrose (100 mg), and Water for Injection USP, to pH 6.0.
Each 150 mg/mL pre-filled pen or pre-filled syringe contains 150 mg alirocumab, histidine (6 mM), polysorbate 20 (0.1 mg), sucrose (100 mg), and Water for Injection USP, to pH 6.0.
Clinical Pharmacology
Mechanism of Action
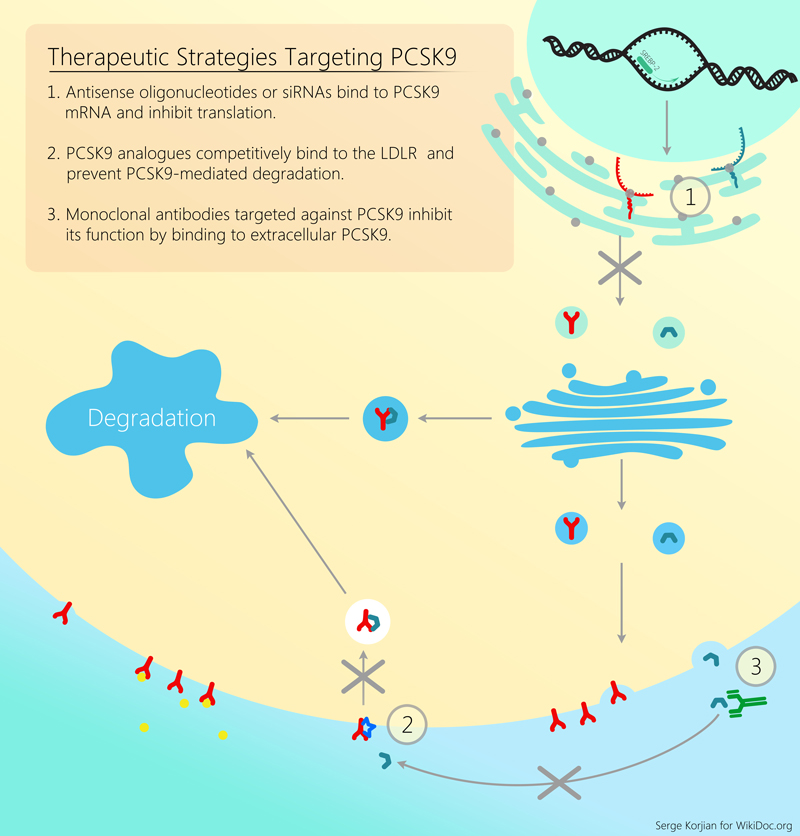
Alirocumab is a human monoclonal antibody that binds to proprotein convertase subtilisin kexin type 9 (PCSK9). PCSK9 binds to the low-density lipoprotein receptors (LDLR) on the surface of hepatocytes to promote LDLR degradation within the liver. LDLR is the primary receptor that clears circulating LDL, therefore the decrease in LDLR levels by PCSK9 results in higher blood levels of LDL-C. By inhibiting the binding of PCSK9 to LDLR, alirocumab increases the number of LDLRs available to clear LDL, thereby lowering LDL-C levels.
Pharmacodynamics
Alirocumab reduced free PCSK9 in a concentration-dependent manner. Following a single subcutaneous administration of alirocumab 75 or 150 mg, maximal suppression of free PCSK9 occurred within 4 to 8 hours. Free PCSK9 concentrations returned to baseline when alirocumab concentrations decreased below the limit of quantitation.
Pharmacokinetics
Absorption
After subcutaneous (SC) administration of 75 mg to 150 mg alirocumab, median times to maximum serum concentrations (tmax) were 3–7 days. The pharmacokinetics of alirocumab after single SC administration of 75 mg into the abdomen, upper arm, or thigh were similar. The absolute bioavailability of alirocumab after SC administration was about 85% as determined by population pharmacokinetics analysis. A slightly greater than dose proportional increase was observed, with a 2.1- to 2.7-fold increase in total alirocumab concentrations for a 2-fold increase in dose. Steady state was reached after 2 to 3 doses with an accumulation ratio of about 2-fold.
Distribution
Following IV administration, the volume of distribution was about 0.04 to 0.05 L/kg indicating that alirocumab is distributed primarily in the circulatory system.
Metabolism and Elimination
- Specific metabolism studies were not conducted, because alirocumab is a protein. Alirocumab is expected to degrade to small peptides and individual amino acids. In clinical studies where alirocumab was administered in combination with atorvastatin or rosuvastatin, no relevant changes in statin concentrations were observed in the presence of repeated administration of alirocumab, indicating that cytochrome P450 enzymes (mainly CYP3A4 and CYP2C9) and transporter proteins such as P-gp and OATP were not affected by alirocumab.
- Two elimination phases were observed for alirocumab. At low concentrations, the elimination is predominately through saturable binding to target (PCSK9), while at higher concentrations the elimination of alirocumab is largely through a non-saturable proteolytic pathway.
- Based on a population pharmacokinetic analysis, the median apparent half-life of alirocumab at steady state was 17 to 20 days in patients receiving alirocumab at subcutaneous doses of 75 mg Q2W or 150 mg Q2W.
Specific Populations
A population pharmacokinetic analysis was conducted on data from 2799 subjects. Age, body weight, gender, race, and creatinine clearance were found not to significantly influence alirocumab pharmacokinetics. No dose adjustments are recommended for these demographics.
Pediatric
Alirocumab has not been studied in pediatric patients.
Renal Impairment
Since monoclonal antibodies are not known to be eliminated via renal pathways, renal function is not expected to impact the pharmacokinetics of alirocumab.
No data are available in patients with severe renal impairment.
Hepatic Impairment
Following administration of a single 75 mg SC dose, alirocumab pharmacokinetic profiles in subjects with mild and moderate hepatic impairment were similar to those in subjects with normal hepatic function.
No data are available in patients with severe hepatic impairment.
Drug-Drug Interactions
The median apparent half-life of alirocumab is reduced to 12 days when administered with a statin; however, this difference is not clinically meaningful and does not impact dosing recommendations.
Non Clinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with alirocumab. The mutagenic potential of alirocumab has not been evaluated; however, monoclonal antibodies are not expected to alter DNA or chromosomes.
There were no adverse effects on surrogate markers of fertility (e.g., estrous cyclicity, testicular volume, ejaculate volume, sperm motility, or total sperm count per ejaculate) in a 6-month chronic toxicology study in sexually-mature monkeys subcutaneously administered at 5, 15, and 75 mg/kg/week at systemic exposures up to 103-fold the 150 mg every two weeks subcutaneous clinical dose based on serum AUC. In addition, there were no adverse alirocumab-related anatomic pathology or histopathology findings in reproductive tissues in rat or monkey toxicology studies at systemic exposures up to 11-fold and 103-fold respectively, in the 6-month studies, compared to clinical systemic exposure following a 150 mg every two weeks dose, based on serum AUC.
Animal Toxicology and/or Pharmacology
During a 13-week toxicology study of 75 mg/kg once weekly alirocumab in combination with 40 mg/kg once daily atorvastatin in adult monkeys, there were no effects of Alirocumab on the humoral immune response to keyhole limpet hemocyanin (KLH) after one to two months at exposures 100-fold greater than the exposure at the maximum recommended human dose of 150 mg every two weeks, based on AUC.
Clinical Studies
The efficacy of Alirocumab was investigated in five double-blind placebo-controlled trials that enrolled 3499 patients; 36% were patients with heterozygous familial hypercholesterolemia (HeFH) and 54% were non-FH patients who had clinical atherosclerotic cardiovascular disease. Three of the five trials were conducted exclusively in patients with HeFH. All patients were receiving a maximally tolerated dose of a statin, with or without other lipid-modifying therapies. In the trials that enrolled patients with HeFH, the diagnosis of HeFH was made either by genotyping or clinical criteria ("definite FH" using either the Simon Broome or WHO/Dutch Lipid Network criteria). All trials were at least 52 weeks in duration with the primary efficacy endpoint measured at week 24 (mean percent change in LDL-C from baseline).
Three studies used an initial dose of 75 mg every 2 weeks (Q2W) followed by criteria-based up-titration to 150 mg Q2W at week 12 for patients who did not achieve their pre-defined target LDL-C at week 8. The majority of patients (57% to 83%) who were treated for at least 12 weeks did not require up-titration. Two studies used only a 150 mg Q2W dose.
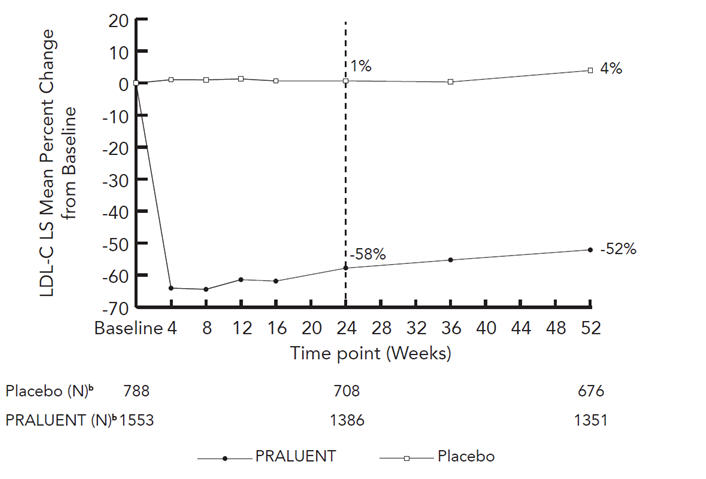
Study 1 was a multicenter, double-blind, placebo-controlled trial that randomly assigned 1553 patients to Alirocumab 150 mg Q2W and 788 patients to placebo. All patients were taking maximally tolerated doses of statins with or without other lipid-modifying therapy, and required additional LDL-C reduction. The mean age was 61 years (range 18–89), 38% were women, 93% were Caucasian, 3% were Black, and 5% were Hispanic/Latino. Overall, 69% were non-FH patients with clinical atherosclerotic cardiovascular disease and 18% had HeFH. The average LDL-C at baseline was 122 mg/dL.
The proportion of patients who prematurely discontinued study drug prior to the 24-week endpoint was 8% among those treated with Alirocumab and 8% among those treated with placebo.
At week 24, the treatment difference between Alirocumab and placebo in mean LDL-C percent change was -58% (95% CI: -61%, -56%; p-value: <0.0001).
| Treatment Group | LDL-C | Total-C | Non-HDL-C | Apo B |
|---|---|---|---|---|
| Week 24 (Mean Percent Change from Baseline) | ||||
| Placebo | 1 | 0 | 1 | 1 |
| Alirocumab
(150 mg) |
-58 | -36 | -49 | -50 |
| Difference from placebo (LS Mean)
(95% CI) |
-58
(-61, -56) |
-36
(-37, -34) |
-50
(-52, -47) |
-51
(-53, -48) |
| *Difference is PRALUENT minus Placebo
†A pattern-mixture model approach was used with multiple imputation of missing post-treatment values based on a subject's own baseline value and multiple imputation of missing on-treatment values based on a model including available ontreatment values. | ||||
Study 2 was a multicenter, double-blind, placebo-controlled trial that randomly assigned 209 patients to Alirocumab and 107 patients to placebo. Patients were taking maximally tolerated doses of statins with or without other lipid-modifying therapy, and required additional LDL-C reduction.
The mean age was 63 years (range 39–87), 34% were women, 82% were Caucasian, 16% were Black, and 11% were Hispanic/Latino. Overall 84% had clinical atherosclerotic cardiovascular disease. Mean baseline LDL-C was 102 mg/dL.
The proportion of patients who prematurely discontinued study drug prior to the 24-week endpoint was 11% among those treated with Alirocumab and 12% among those treated with placebo.
At week 12, the mean percent change from baseline in LDL-C was -45% with Alirocumab compared to 1% with placebo, and the treatment difference between Alirocumab 75mg Q2W and placebo in mean LDL-C percent change was -46% (95% CI: -53%, -39%).
At week 12, if additional LDL-C lowering was required based on pre-specified LDL-C criteria, Alirocumab was up-titrated to 150 mg Q2W for the remainder of the trial. At week 24, the mean percent change from baseline in LDL-C was -44% with Alirocumab and -2% with placebo, and the treatment difference between Alirocumab and placebo in mean LDL-C percent change was -43% (95% CI: -50%, -35%; p-value: <0.0001). The dose was up-titrated to 150 mg Q2W in 32 (17%) of 191 patients treated with Alirocumab for at least 12 weeks.
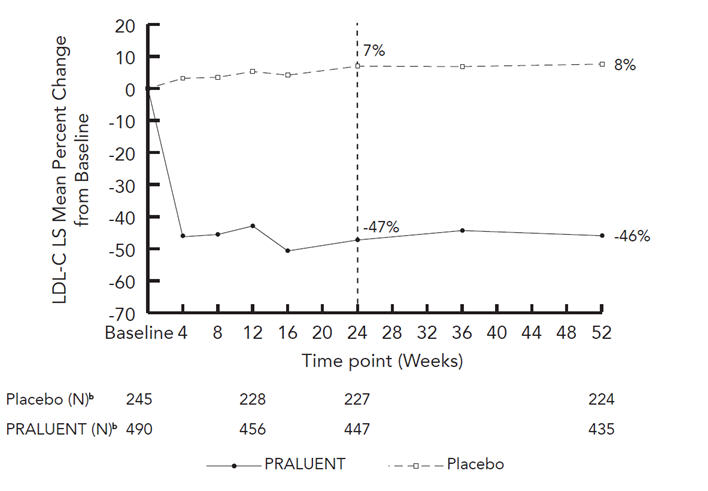
Studies 3 and 4 were multicenter, double-blind, placebo-controlled trials that, combined, randomly assigned 490 patients to Alirocumab and 245 patients to placebo. The trials were similar with regard to both design and eligibility criteria. All patients had HeFH, were taking a maximally tolerated dose of statin with or without other lipid-modifying therapy, and required additional LDL-C reduction. The mean age was 52 years (range 20–87), 45% were women, 94% were Caucasian, 1% were Black, and 3% were Hispanic/Latino. Overall, 45% of these patients with HeFH also had clinical atherosclerotic cardiovascular disease. The average LDL-C at baseline was 141 mg/dL.
Considering both trials together, the proportion of patients who prematurely discontinued study drug prior to the 24-week endpoint was 6% among those treated with Alirocumab and 4% among those treated with placebo.
At week 12, the treatment difference between Alirocumab 75 mg Q2W and placebo in mean LDL-C percent change was -48% (95% CI: -52%, -44%).
At week 12, if additional LDL-C lowering was required based on pre-specified LDL-C criteria, Alirocumab was up-titrated to 150 mg Q2W for the remainder of the trials. At week 24, the mean treatment difference between Alirocumab and placebo in mean LDL-C percent change from baseline was -54% (95% CI: -59%, -50%; p-value: <0.0001). The dose was up-titrated to 150 mg Q2W in 196 (42%) of 469 patients treated with Alirocumab for at least 12 weeks. The LDL-C-lowering effect was sustained to week 52.
| Treatment Group | LDL-C | Total-C | Non-HDL-C | Apo B |
|---|---|---|---|---|
| Week 12 (Mean Percent Change from Baseline) | ||||
| Placebo | 5 | 4 | 5 | 2 |
| Alirocumab (75 mg) | -43 | -27 | -38 | -34 |
| Difference from placebo (LS Mean) (95% CI) | -48
(-52, -44) |
-31
(-34, -28) |
-42
(-46, -39) |
-36
(-39, -33) |
| Week 24 (Mean Percent Change from Baseline) | ||||
| Placebo | 7 | 5 | 7 | 2 |
| Alirocumab (75/up150 mg‡) | -47 | -30 | -42 | -40 |
| Difference from placebo (LS Mean) (95% CI) | -54
(-59, -50) |
-36
(-39, -33) |
-49
(-53, -45) |
-42
(-45, -39) |
| ||||
Study 5 was a multicenter, double-blind, placebo-controlled trial that randomly assigned 72 patients to Alirocumab 150 mg Q2W and 35 patients to placebo. Patients had HeFH with a baseline LDL-C ≥160 mg/dL while taking a maximally tolerated dose of statin with or without other lipid-modifying therapy. The mean age was 51 years (range 18–80), 47% were women, 88% were Caucasian, 2% were Black, and 6% were Hispanic/Latino. Overall, 50% had clinical atherosclerotic cardiovascular disease. The average LDL-C at baseline was 198 mg/dL.
The proportion of patients who discontinued study drug prior to the 24-week endpoint was 10% among those treated with Alirocumab and 0% among those treated with placebo.
At week 24, the mean percent change from baseline in LDL-C was -43% with Alirocumab and -7% with placebo, and the treatment difference between Alirocumab and placebo in mean LDL-C percent change was -36% (95% CI: -49%, -24%; p-value: <0.0001).
How Supplied/Storage and Handling
Alirocumab is a clear, colorless to pale yellow solution, supplied in single-dose pre-filled pens and single-dose pre-filled glass syringes. Each pre-filled pen or pre-filled syringe of Alirocumab is designed to deliver 1 mL of 75 mg/mL or 150 mg/mL solution.
Alirocumab is available in cartons containing 1 or 2, pre-filled pens and 1 or 2, pre-filled syringes.
| Pack Size | 75 mg/mL Pre-Filled Pen | 150 mg/mL Pre-Filled Pen |
|---|---|---|
| Pack of 2 pens | NDC 0024-5901-01 | NDC 0024-5902-01 |
| Pack of 1 pen | NDC 0024-5901-02 | NDC 0024-5902-02 |
| Pack Size | 75 mg/mL Pre-Filled Syringe | 150 mg/mL Pre-Filled Syringe |
|---|---|---|
| Pack of 2 syringes | NDC 0024-5903-01 | NDC 0024-5904-01 |
| Pack of 1 syringe | NDC 0024-5903-02 | NDC 0024-5904-02 |
Store in a refrigerator at 36°F to 46°F (2°C to 8°C) in the outer carton in order to protect from light.
Do NOT freeze. Do NOT expose to extreme heat. Do NOT shake.
Patient Counseling Information
For patient counseling information about alirocumab click here.
Drug Packaging and Label
Pen
Injection
Major Trials
Synopsis
| Trial Name | Year | NCT Identifier | Phase | N | Study Population | Primary Endpoint | Secondary Endpoints | Safety Endpoints | Randomization | Intervention
Arm |
Control
Arm(s) |
Study Duration | Follow up | Main Study Findings | Other Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT 01288469 | II | 92 | |||||||||||||
| Linked to FH | NCT 01266876 | II | 77 | ||||||||||||
| NCT 01288443 | II | 183 | |||||||||||||
| COMBO I | 2012-2013 | NCT 01644175 | III | 316 |
|
% change in LDL at week 24 in ITT population | % change in LDL at different timepoints in "on-treatment" population | AE reporting | 2:1 | 75mg SQ Alirocumab Q2W
increased to 150 mg if LDL ≥70mg/dl by week 12 |
Matched
Placebo |
52 weeks | 8 weeks | -48.2% vs. -2.3% (Δ:45.9%; 95% CI: 39.3-52.5; p<0.0001) |
|
| COMBO II | 2012-2013 | NCT 01644188 | III | 720 |
|
% change in LDL at week 24 in ITT population | % change in LDL at week 12(ITT or OT), 24 (OT), 52 (ITT or OT) | AE reporting | 2:1 | 75mg SQ Alirocumab Q2W
increased to 150 mg if LDL ≥70mg/dl by week 12 |
Ezetimibe 10mg PO QD | 104 weeks | 8 weeks | -50.6% vs. -20.7% (Δ:29.8%; 95% CI: 25.3-34.4; p<0.0001) |
|
| ALTERNATIVE | 2012-2013 | NCT 01709513 | III | 314 |
|
% change in LDL at week 24 in ITT population compared to ezetimibe | % change in LDL at week 12 and 24 in "on-treatment" population compared to ezetimibe | All Adverse events during the study and the optional open label study extension | 2:2:1 | 75mg SQ Alirocumab Q2W |
OR
|
24 weeks | 8 week follow up with option to extend to 3 year open label study | -45.0% vs. -14.6% (Δ:30.4%; 95% CI: 24.2-36.6; p<0.0001) |
|
| MONO | 2012-2013 | NCT 01644474 | III | 103 |
|
% change in LDL at week 24 in ITT population | % change in LDL at week 12 in ITT population | AE reporting up to 10 weeks after last dose | 1:1 | 75mg SQ Alirocumab Q2W
increased to 150 mg if LDL ≥100 mg/dl by week 12 (70mg/dl was utilized instead of 100mg/dl due to admin error) |
Ezetimibe 10mg PO QD | 24 weeks | 8 weeks | -47.2% vs. -15.6% (Δ:31.6%; 95% CI: 23.0-40.2; p<0.0001) |
|
| FHI | 2012-2014 | NCT 01623115 | III | 486 |
|
% change in LDL at week 24 in ITT population |
|
AE reporting | 2:1 | 75mg SQ Alirocumab Q2W
increased to 150 mg if LDL ≥70mg/dl by week 12 |
Placebo | 78 weeks | 8 weeks | -48.8% vs. 9.1% (Δ:57.9%; 95% CI: 52.6-63.3; p<0.0001) |
|
| FHII | 2012-2014 | NCT 01709500 | III | 249 | -48.7% vs. 2.8% (Δ:51.4%; 95% CI: 44.8-58.1; p<0.0001) | ||||||||||
| Long Term | 2012-2014 | NCT 01507831 | III | 2314 |
|
% change in LDL at week 24 in ITT population | % change in LDL at week 12 and 24 in "on-treatment" population | Adverse Events up to 10 weeks after last dose | 2:1 | Single 150mg SQ Alirocumab Q2W | Matched
Placebo |
78 weeks | 8 weeks | -61.0% vs. 0.8% (Δ:61.9%; 95% CI: 59.4-64.3; p<0.001) | Similar rates of any Adverse event between the groups. |
| OPTIONS I | 2012-2014 | NCT 01730040 | III | 355 |
|
% change in LDL at week 24 in ITT population
compared to any of the five control arms |
% change in LDL at week 12 and 24 in "on-treatment" population | AE reporting | 1:1:1
(for 20mg baseline atorvastatin) N=169 |
Add on therapy
to 20mg ATV: 75mg SQ Alirocumab Q2W increased to 150 mg if LDL ≥70mg/dl (with CVD) or ≥100mg/dl (without CVD) by week 12 |
OR
|
24 weeks | 8 weeks | -44.1 vs. -20.5 vs. -5.0 (ALI vs. EZE vs. ATV) |
|
| 1:1:1:1
(for 40mg baseline atorvastatin) N=186 |
Add on therapy
to 40mg ATV: 75mg SQ Alirocumab Q2W increased to 150 mg if LDL ≥70mg/dl (with CVD) or ≥100mg/dl (without CVD) by week 12 |
OR
OR
|
-54.0 vs. -22.6 vs. -4.8 vs. -21.4 (ALI vs. EZE vs. ATV vs. RSV) |
| |||||||||||
| OPTIONS II | 2012-2014 | NCT 01730053 | III | 305 |
|
% change in LDL at week 24 in ITT population
compared to any of the four control arms |
% change in LDL at week 24 in "on-treatment" population
compared to any of the four control arms |
AE reporting | 1:1:1 | Add on therapy
to RSV: 75mg SQ Alirocumab Q2W increased to 150 mg if LDL ≥70mg/dl (with CVD) or ≥100mg/dl (without CVD) by week 12 |
OR
|
24 weeks | 8 weeks | -50.6% vs. -14.4% vs. -16.3%
(ALI vs. EZE vs. RSV) |
|
OR
|
-36.3%
vs. -11.0% vs. -15.9% (ALI vs. EZE. vs. RSV) |
| |||||||||||||
| CHOICE I | 2013-2014 | NCT 01926782 | III | 803 |
|
AND
|
|
AE reporting | 4:2:1 | 300mg SQ Alirocumab Q4W decreased to 150 mg Q4W if LDL ≥70mg/dl (with very high CVD risk) or ≥100mg/dl (with moderate CVD) by week 12 |
75mg SQ Alirocumab Q2W increased to 150 mg if LDL ≥70mg/dl (with very high CVD risk) or ≥100mg/dl (with moderate CVD risk) by week 12 OR Placebo |
48 weeks | 8 weeks |
|
|
| CHOICE II | 2013-2014 | NCT 02023879 | III | 233 |
|
% change in LDL at week 24 in ITT population compared to placebo |
|
AE reporting | 2:1:1
(75mgQ2W:150mgQ4W:Placebo) |
150 mg SQ alirocumab Q4W increased to 150 mg Q2W (300mg total) in patients if LDL ≥70mg/dl (with CVD) or ≥100mg/dl (without CVD) at 12 week |
|
24 weeks | 8 weeks
with option to extend |
-51.7% vs 4.7% (Δ:56.4%; 95% CI; p<0.0001) |
|
| HIGH FH | 2012-2015 | NCT 01617655 | III | 107 |
|
% change in LDL at week 24 in ITT population |
|
AE reporting | 2:1 | 150 mg SQ Alirocumab Q2W | Placebo | 78 weeks | 8 weeks | -45.7% vs -6.6% (Δ:39.1%; p<0.0001) |
|
| JAPAN | 2014-2015 | NCT 02107898 | III | 216 |
|
% change in LDL at week 24 in ITT population |
|
AE reporting | 2:1 | 75 mg SQ Alirocumab Q2W increased to 150 mg Q2W in patients if LDL≥100mg/dl (without CVD) at 12 week | Placebo | 52 weeks | 8 weeks | -62.5% vs 1.6% (Δ:64.1%; 95% CI; 59.8-68.5; p<0.0001) |
|
| ESCAPE | 2015 | NCT 02326220 | III | 62 |
|
Rate of apheresis during 12 week period from week 7-18 compared to number of planned apheresis tx |
|
AE reporting up to 10 weeks after last dose | 2:1 | 150 mg SQ Alirocumab Q2W | Placebo | 18 weeks | 8 weeks | 75% reduction in rate of apheresis weeks 7-18 (ALI vs. Placebo)
HR:67%-83%, p<0.0001 |
|
Phase II Trials
Safety and efficacy of atorvastatin with or without alirocumab in primary hypercholesterolemia (NCT 01288469)
This randomized, double-blind, placebo-controlled phase 2 trial of 92 patients with LDL-C≥100 mg/dL after treatment with 10 mg of atorvastatin for at least 7 weeks randomized patients to 8 weeks of therapy with either 80 mg of atorvastatin daily plus 150 mg SC alirocumab once every 2 weeks, 10 mg of atorvastatin daily plus 150 mg alirocumab once every 2 weeks, or 80 mg of atorvastatin daily plus SC placebo once every 2 weeks. The trial demonstrated a significant 73.2% reduction from baseline serum LDL-C cholesterol with 80 mg of atorvastatin plus alirocumab compared with 17.3% with the 80 mg atorvastatin plus placebo. Ninety percent of the patients who received alirocumab reached LDL-C concentrations lower than 70 mg/dL compared with 17% of those receiving atorvastatin alone. There were no significant safety signals and the drug was well tolerated.[2]
Safety and efficacy in heterozygous familial hypercholesterolaemia with ongoing stable-dose statin with or without ezetimibe therapy (NCT 01266876)
This randomized, double-blind, placebo-controlled phase 2 trial of 77 adults with heterozygous familial hypercholesterolaemia and LDL-C concentrations of ≥100 mg/dL or higher on stable diet and statin dose, with or without ezetimibe therapy were randomized (1:1:1:1:1) to 5 different treatment arms for 12 weeks: 150 mg SC alirocumab every 4 weeks, 200 mg SC alirocumab every 4 weeks, 300 mg SC alirocumab every 4 weeks, 150 mg SC alirocumab every 2 weeks, or SC placebo. Randomization was stratified by baseline use of ezetimibe. Alirocumab demonstrated a significant reduction in LDL-C at week 12 (28.9%, 31.5%, and 42.5% for the 150, 200, and 300 mg every 4 weeks respectively, and 67.9% for the 150 mg every 2 weeks dose, compared with 10.7% in the placebo arm) with no significant safety signal. There were no increases >3 x ULN in hepatic transaminases or creatine kinase (CK).[3]
Safety and efficacy in primary hypercholesterolemia with ongoing stable atorvastatin therapy (NCT 01288443)
This randomized, double-blind, placebo-controlled phase 2 trial of 183 patients with LDL-C ≥100 mg/dL on stable-dose atorvastatin for 6 or more weeks randomized patients to a 12 week treatment course in either one of 6 arms (1:1:1:1:1:1): subcutaneous (SC) placebo every 2 weeks, 50 mg SC alirocumab every 2 weeks, 100 mg SC alirocumab every 2 weeks, 150 mg SC alirocumab every 2 weeks, 200 mg SC alirocumab every 4 weeks alternating with placebo, or 300 mg SC alirocumab every 4 weeks alternating with placebo. Alirocumab demonstrated a significant dose-related reduction in serum LDL-C (40%, 64%, and 72% with 50, 100, and 150 mg respectively, and 43% and 48% with 200 and 300 mg respectively compared with 5% in placebo) with no major safety signals. [4]
Phase III Trials
ODYSSEY - COMBO I
COMBO I was a randomized, double blind, trial that randomized 316 adult patients with moderate to very high CV risk and elevated LDL-C despite maximal statin use to either SC alirocumab 75mg every 2 weeks for 52 weeks or a matched placebo. Alirocumab dose was increased at week 12 to 150mg every two weeks if LDL targets failed to be met by week 8. Alirocumab was generally well tolerated, with no reported safety signals in the 75mg or 150mg dose. Alirocumab treatment was associated with a significantly higher reduction in LDL-C from baseline to 24 weeks ( -48.2% for alirocumab vs. -2.3% for placebo; Δ:45.9%; 95% CI: 39.3-52.5; p<0.0001). Patients on alirocumab were more likely to reach LDL targets (75% for alirocumab vs. 9% for placebo). Patients on alirocumab demonstrated a substantial drop in LDL-C within the first 4 weeks, which was sustained to week 52.
ODYSSEY - COMBO II
COMBO II was a randomized, double-blind, double-dummy, trial that randomized 720 patients with elevated CV risk and LDL-C despite maximal statins use to either SC alirocumab 75 mg every 2 weeks (and PO placebo) or PO ezetimibe 10 mg daily (and SC placebo). Alirocumab was generally well tolerated, with no reported safety signals. Alirocumab treatment was associated with a significantly higher reduction in mean LDL-C values from baselines at week 24 (50.6 ± 1.4% for alirocumab vs. 20.7 ± 1.9% for ezetimibe; P < 0.0001). Patients on alirocumab were more likely to achieve LDL-C <1.8 mmol/L (77.0% vs. 45.6%; P < 0.0001). At week 24, mean LDL-C levels were 1.3 ± 0.04 mmol/L among patients receiving alirocumab, and 2.1 ± 0.05 mmol/L among patients receiving ezetimibe.[5]
ODYSSEY -ALTERNATIVE
ALTERNATIVE was a randomized, double blind, double dummy trial that randomized 314 adult patients with moderate to very high CV risk and who underwent 2 weeks washout from lipid lowering therapies in a 2:1:1 ratio to 75mg alirocumab every two weeks, or 10mg oral ezetimibe daily, or 20mg oral atorvastatin daily for 24 weeks. Alirocumab was associated with the lowest rates of skeletal muscle associated AEs, followed by ezetimibe and atorvastatin, respectively. Alirocumab was generally well tolerated, with no significant safety signals. Alirocumab treatment was associated with a significantly higher reduction in mean LDL-C from baseline to week 24 compared to ezetimibe (-45.0% for alirocumab vs. -14.6% for ezetimibe; Δ:30.4%; 95% CI: 24.2-36.6; p<0.0001). Patients on alirocumab were more likely to achieve LDL targets (41.9% vs. 4.4%). Alirocumab patients demonstrated a substantial drop in LDL-C in the first 4 weeks, which was sustained for 24 weeks.
ODYSSEY - MONO
ODYSSEY- MONO was a randomized, double blind, double dummy trial that enrolled 103 adult patients with a 10 year fatal CV risk of 1.00% to 4.99% and LDL-C>100mg/dl. Patients had no established history of coronary heart disease or any CHD risk equivalents. Additionally, patients were not receiving lipid lowering therapy for 4 weeks prior to randomization. Patients were randomized to receive 75mg of SC alirocumab twice weekly or oral ezetimibe 10mg once daily. Alirocumab dose was increased at week 12 to 150mg every two weeks if LDL targets failed to be met by week 8. Alirocumab treatment was associated with a significantly higher reduction in mean LDL-C from baseline to week 24 compared to ezetimibe (-47.2% for alirocumab vs. -15.6% for ezetimibe; Δ:31.6%; 95% CI: 23.0-40.2; p<0.0001). Alirocumab patients demonstrated a substantial drop in LDL-C in the first 4 weeks, which was sustained for 24 weeks. Alirocumab was well tolerated with no safety signals.
ODYSSEY - FH I & FH II
ODYSSEY FHI and FH II were multicenter, double-blind, placebo-controlled trials that enrolled a total of 735 heterozygous familial hypercholesterolemia patients and randomized them to either SC alirocumab 75-150 mg every 2 weeks or matching placebo for a total of 78 weeks, on top of a background of lipid lowering therapy.The primary endpoint was the prrcent change in LDL-C from baseline to week 24. Alirocumab administration was associated with a 57.9% reduction compared to placebo in the FH I population (P<0.0001), and a 51.4% reduction compared to placebo in the FH II population (P<0.0001). Alirocumab was well tolerated and there were no safety concerns.
ODYSSEY - Long Term
Long term was a randomized, double-blind, placebo-controlled trial that enrolled 2341 patients at high risk for CV events with baseline LDL-C ≥70 mg/dL. Patients were randomized in 2:1 ratio to receive either 150mg SC alirocumab or equivalent placebo once every 2 weeks for 78 weeks. The primary efficacy end point was the percentage change in calculated LDL cholesterol level from baseline to week 24. Alirocumab treatment was associated with a 62% reduction in LDL-C compared to placebo (P<0.001). In an exploratory analysis, alirocumab was associated with a significant reduction in the rate of major adverse cardiovascular events (1.7% vs. 3.3%; hazard ratio, 0.52; 95% confidence interval, 0.31 to 0.90; nominal P=0.02). Alirocumab treatment was associated with a significantly higher incidence of myalgia (5.4% vs. 2.9%; P=0.006). [6]
ODYSSEY - OPTIONS I
OPTIONS I was a randomized, double blind, trial that enrolled 355 patients with high or very high CVD risk and elevated LDL-C. All patients were receiving 20 or 40 mg of atorvastatin for at least 4 weeks prior to study enrollment. Patients were stratified by their baseline dose of 20mg atorvastatin vs. 40mg atorvastatin prior to randomization. Patients receiving 20mg of atorvastatin were randomized in a 1:1:1 ratio to receive add-on therapy of 75mg SC alirocumab twice weekly, or add-on therapy of 10mg oral ezetimibe once daily, or doubling the atorvastatin dose to 40mg once daily. Patients receiving a baseline 40mg of atorvastatin were randomized in a 1:1:1:1 ratio to receive add-on therapy of 75mg SC aloricumab twice weekly, or add-on therapy of 10mg oral ezetimibe once daily, or doubling atorvastatin dose to 80mg once daily, or switching to 40mg oral rosuvastatin once daily. Patients were followed for 24 weeks.
Among patients receiving a 20mg baseline dose of atorvastatin , add-on therapy of alirocumab was associated with a 44.1% reduction in mean LDL-C compared to 20.5% for ezetimibe, and 5.0% for 40mg atorvastatin. Patients on alirocumab add-on treatment were most likely to achieve LDL-C targets (79.2% for alirocumab vs. 50.3% for ezetimibe vs. 16.0% for 40mg atorvastatin).
Among patients receiving 40mg baseline dose of atorvastatin, add-on therapy of alirocumab was associated with a 54.0% reduction in mean LDL-C compared to 22.6% for ezetimibe, 4.8% for atorvastatin, and 21.5% for rosuvastatin. Patients on alirocumab add-on treatment were most likely to achieve LDL-C targets (77.2% for alirocumab vs. 54.2% for ezetimibe vs. 10.2% for 80mg atorvastatin, and 42.2% for rosuvastatin).
Alirocumab was well tolerated and showed no safety signals. Regardless of baseline dose of atorvastatin, patients experienced a substantial drop in mean LDL-C within the first 4 weeks which was sustained for 24 weeks.
ODYSSEY - OPTIONS II
OPTIONS II was a randomized, double blind, trial that enrolled 305 patients with high or very high CVD risk and elevated LDL-C. All patients were receiving 10 or 20 mg of rosuvastatin for at least 4 weeks prior to study enrollment. Patients were stratified by their baseline dose of 10mg rosuvastatin vs. 20mg rosuvastatin prior to randomization. Patients receiving 10mg of rosuvastatin were randomized in a 1:1:1 ratio to receive add-on therapy of 75mg SC alirocumab twice weekly, or add-on therapy of 10mg oral ezetimibe once daily, or doubling the rosuvastatin dose to 20mg once daily. Patients receiving a baseline 20mg of rosuvastatin were randomized in a 1:1:1 ratio to receive add-on therapy of 75mg SC aloricumab twice weekly, or add-on therapy of 10mg oral ezetimibe once daily, or doubling rosuvastatin dose to 40mg once daily, Patients were followed for 24 weeks.
Among patients receiving a 10mg baseline dose of rosuvastatin , add-on therapy of alirocumab was associated with a 50.6% reduction in mean LDL-C compared to 14.4% for ezetimibe, and 16.3% for 20mg rosuvastatin. Patients on alirocumab add-on treatment were most likely to achieve LDL-C targets (77.8% for alirocumab vs. 43.1% for ezetimibe vs. 31.3% for 20mg rosuvastatin).
Among patients receiving 20mg baseline dose of rosuvastatin, add-on therapy of alirocumab was associated with a 36.3% reduction in mean LDL-C compared to 11.0% for ezetimibe, and 15.9% for rosuvastatin. Patients on alirocumab add-on treatment were most likely to achieve LDL-C targets (60.1% for alirocumab vs. 43.6% for ezetimibe vs. 29.9% for 40mg rosuvastatin). Results of the 20mg group missed the mark for statistical significance.
Alirocumab was well tolerated and showed no safety signals.
ODYSSEY - CHOICE I
CHOICE I was a randomized, double blind, placebo controlled clinical trial that enrolled 803 adult patients with uncontrolled hypercholesterolemia and moderate to very high CVD risk receiving maximum dose of statins, have skeletal muscle symptoms associated with statin use or discontinued statin use. Patients were randomized in a 4:2:1 ratio to receive 300mg SC alirocumab every 4 weeks, or 75mg SC alirocumab every 2 weeks, or placebo for 48 weeks. Alirocumab treatment was well tolerated and showed no significant safety signals.
Among patients not receiving statins at baseline; those receiving alirocumab demonstrated a 52.7% reduction in mean LDL-C compared to 0.3% in placebo. Among patients receiving statins at baseline; those receiving alirocumab demonstrated a 48.8% reduction in mean LDL-C compared to 0.1% in placebo.
ODYSSEY - CHOICE II
CHOICE II was a randomized, double blind, placebo controlled clinical trial that enrolled 233 adult patients with uncontrolled hypercholesterolemia and discontinued statins. This trial enrolled a large subgroup of patients who previously experienced statin associated myopathy. Patients were randomized in a 2:1:1 ratio to receive 150mg SC alirocumab every four weeks, or placebo, or 75mg SC alirocumab every two weeks. Primary effficacy endpoint was the percent change in mean LDL-C from baseline to week 24 in the ITT population compared to placebo. Alirocumab was well tolerated and demonstrated no safety signals. There was a low incidence of musculoskeletal symptoms across all groups. More injection site reactions were associated with the 150mg dose compared to placebo or the 75mg dose.
Patients receiving alirocumab had a 51.7% mean reduction in LDL-C compared to 4.7% in patients receiving placebo. The 75mg twice weekly dose demonstrated a higher efficacy against placebo compared to the 150mg every 4 weeks dose. More patients achieved target LDL-C levels in the 75mg dose group compared to 150mg and placebo groups (70.3% vs. 63.9% vs. 1.8%, respectively).
ODYSSEY - HIGH FH
HIGH FH was a randomized, double blind, placebo controlled clinical trial that enrolled 107 adult patients with heterozygous familial hypercholesterolemia with poorly controlled cholesterol levels (LDL>150mg/dl) despite maximum stable dose of statins. Patients were randomized in a 2:1 fashion to receive 150mg SC alirocumab every two weeks or placebo for 78 weeks. Alirocumab was well tolerated and demonstrated no safety signals.
Patients on alirocumab had a 45.7% reduction in LDL-C from baseline to week 24 compared to 6.6% reduction in the placebo group. 41.0% of patients in the alirocumab group achieved LDL targets compared to 5.7% in the placebo group.
ODYSSEY - JAPAN
ODYSSEY JAPAN was a randomized, double blind, placebo controlled clinical trial that enrolled 216 adult patients at 31 sites in Japan. Patients had heterozygous familial hypercholesterolemia and those with high cardiovascular risk with a history of coronary artery disease. Patients had uncontrolled hypercholesterolemia with an elevated LDL-C despite lipid lowering therapy. Eligible patients were randomized in a 2:1 fashion to receive 75mg SC alirocumab every two weeks or placebo, for a duration of 52 weeks. Surprisingly, patients receiving placebo experienced more SAEs compared to patients in the alirocumab group (12.5% for placebo and 7.0% for alirocumab). Alirocumab was well tolerated, and no safety concerns were observed.
Patients receiving alirocumab demonstrated a 62.5% reduction in mean LDL-C from baseline to week 24 compared to placebo; 1.6%. This was demonstrated with a substantial drop in the first 4 weeks and sustained for 52 weeks. 96.7% of patients receiving alirocumab achieved LDL-C targets compared to 10.2% of patients in the placebo group.
ODYSSEY - ESCAPE
ESCAPE was a randomized, double blind, placebo-controlled trial of 62 adult patients with heterozygous familial hypercholesterolemia with high cardiovascular risk on previous statin treatment and currently undergoing apheresis therapy every week or two weeks for at least 4 weeks prior to randomization. Patients are randomized in a 2:1 ratio to receive 150mg SQ alirocumab once every two weeks or placebo. Alirocumab was well tolerated and generated no significant safety signal.
Patients receiving alirocumab demonstrated a 75% reduction in the rate of apheresis from weeks 7 to 18 compared to placebo. Patients experienced a substantial drop in LDL-C for the first two weeks and the effect was sustained for 52 weeks. Additionally, patients on alirocumab demonstrated a 50% reduction in rate of apheresis in weeks 15-18, compared to placebo.
Cost-Effectiveness
Doses are administered every two weeks with a cost of $40 a day or $14,600 a year, substantially higher than some generic statins, which can cost as little as $0.10 a day. alirocumab is more expensive to manufacture than statins because it is made in live genetically engineered cells. Manufacturers argue that the drug is cost-effective because it will reduce medical costs of hospitalizations from stroke or myocardial infarction and that the price of the drug reflects its value. alirocumab used in combination with statins can lower cholesterol 40-70% [6] compared to statins that lower LDL an average of 40% [7]. Still, further research into the actual ability of the drug to reduce risk and complications is ongoing. Reduced prices and plans through insurers should help make the drug accessible to patients with lower ability to pay.
Future Investigations
The following trials are currently underway and study designs, rationale, and results are not yet published.
ODYSSEY - OUTCOMES
OUTCOMES will be a randomized, double blind, placebo controlled, clinical trial enrolling 18,000 patients over 40 years of age who are hospitalized for acute coronary syndrome with elevated cardiac biomarkers, or ECG changes consistent with ischemia or infarction, and evidence of obstructive coronary artery disease. Patients must demonstrate inadequate control of artherogenic lipoproteins despite maximal statin regimen. Patients will be randomized in a 1:1 ratio to receive 75mg SQ alirocumab every two weeks or a matched placebo for a period of 2 to 5 years. Alirocumab dose may be increased to 150mg SQ q2w if LDL-C is sustained above 50mg/dL after 4 weeks. Doses of alirocumab may be downtitrated from 150mg q2w to 75mg q2w if LDL-C is below 25mg/dL.
The primary endpoint is time to first of a composite of coronary heart disease death, non-fatal MI, Ischemic stroke, or unstable angina requiring hospitalization. Pre-specified secondary outcomes include time to each component of the primary endpoint. Additional genomic analysis will be performed on a subset of patients who consent to it.
The ODYSSEY Outcomes trial seeks to determine whether clinical outcomes are improved by lowering levels of LDL-C and other atherogenic lipoproteins below those achieved on optimal statin therapy alone. The trial is not designed to explore the safety of sustained, very low LDL-C levels. The trial will determine whether further reduction in cardiovascular risk can be achieved by addition of the monoclonal PCSK9 antibody, alirocumab, resulting in further reduction of LDL-C and other atherogenic lipoproteins.
DM DYSLIPIDEMIA
Efficacy and safety of alirocumab in high cardiovascular risk patients with diabetes.
DM INSULIN
Efficacy and safety of alirocumab in insulin-treated patients with Type 1 or Type 2 diabetes and high cardiovascular risk.
KT
A randomized, double-blind, placebo-controlled, parallel group study to evaluate the efficacy and safety of alirocumab in high cardiovascular risk patients with hypercholesterolemia not adequately controlled with their lipid modifying therapy in South Korea and Taiwan.
References
- ↑ Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. (2013). "Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis". J Am Coll Cardiol. 62 (16): 1401–8. doi:10.1016/j.jacc.2013.07.056. PMID 23973703. Unknown parameter
|month=ignored (help) - ↑ Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA (2012). "Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia". N Engl J Med. 367 (20): 1891–900. doi:10.1056/NEJMoa1201832. PMID 23113833.
- ↑ Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R; et al. (2012). "Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial". Lancet. 380 (9836): 29–36. doi:10.1016/S0140-6736(12)60771-5. PMID 22633824.
- ↑ McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA (2012). "Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy". J Am Coll Cardiol. 59 (25): 2344–53. doi:10.1016/j.jacc.2012.03.007. PMID 22463922.
- ↑ Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R; et al. (2015). "Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial". Eur Heart J. doi:10.1093/eurheartj/ehv028. PMID 25687353.
- ↑ 6.0 6.1 Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M; et al. (2015). "Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events". N Engl J Med. doi:10.1056/NEJMoa1501031. PMID 25773378.
- ↑ Anand SS (2003). "Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. Law MR, Wald NJ, Rudnicka AR. BMJ 2003; 326: 1407-408". Vasc Med. 8 (4): 289–90. PMID 15125495.