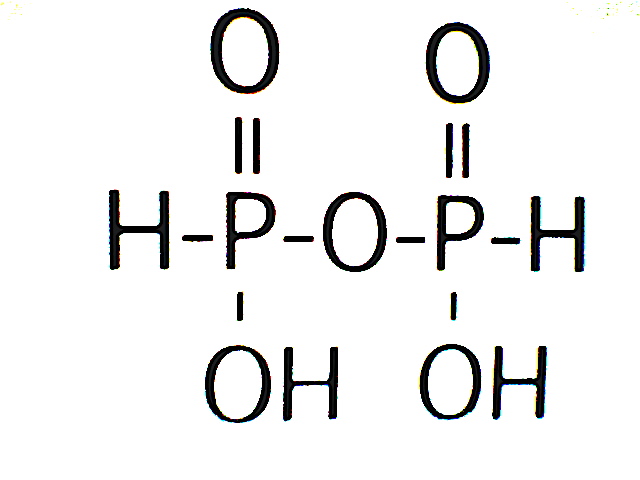Phosphonic acid
|
WikiDoc Resources for Phosphonic acid |
|
Articles |
|---|
|
Most recent articles on Phosphonic acid Most cited articles on Phosphonic acid |
|
Media |
|
Powerpoint slides on Phosphonic acid |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Phosphonic acid at Clinical Trials.gov Trial results on Phosphonic acid Clinical Trials on Phosphonic acid at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Phosphonic acid NICE Guidance on Phosphonic acid
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Phosphonic acid Discussion groups on Phosphonic acid Patient Handouts on Phosphonic acid Directions to Hospitals Treating Phosphonic acid Risk calculators and risk factors for Phosphonic acid
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Phosphonic acid |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: Henry A. Hoff
Overview
In inorganic chemistry, phosphonic acid is a phosphorus oxoacid with a formula of H3PO3, more commonly known as phosphorous acid. It exists in solution as two tautomers, the major one being HP(O)(OH)2 and the minor one P(OH)3. The former is sometimes termed phosphonic acid, with the latter designated as phosphorous acid. Sometimes confusingly, both these names are also used to refer to H3PO3 in general, i.e. both tautomers.
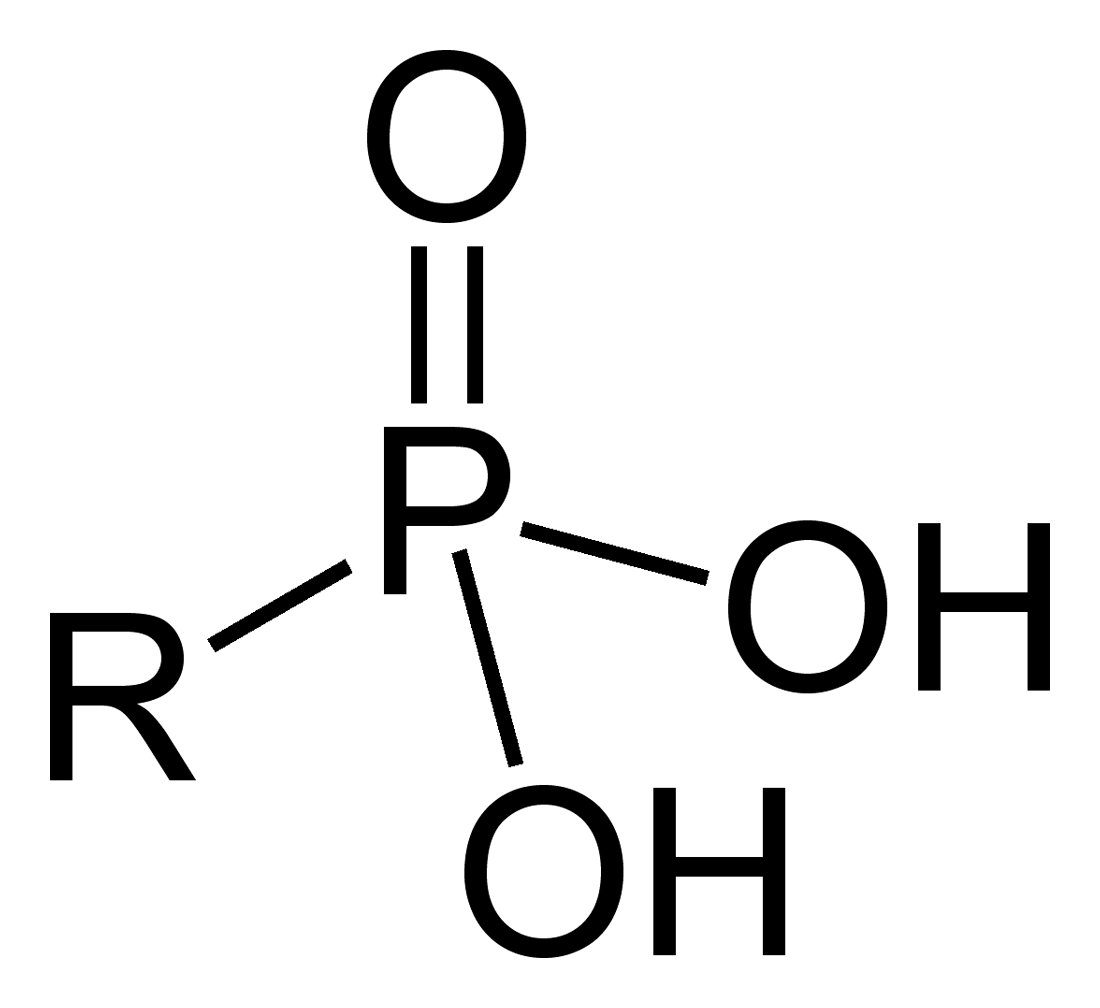
In organic chemistry, a phosphonic acid is a compound with the general formula RP(O)(OH)2.
An example of an organic phosphonic acid is Foscarnet.
An oligophosphonic acid refers to a few molecules of phosphonic acid condensed into a molecule with the loss of water.
A general formula for such oligophosphonic acids is (HPO)nOn-1(OH)2, where n = 2, 3, 4, etc., oligo-. A polyphosphonic acid can have dozens of such phosphonic acid units condensed in a row with the loss of H2O for each unit added on.
An example that incorporates triphosphonic acid: ethane-1,1,2-triphosphonic acid[1]. In some phosphonic anhydrides (RPO2)3, R can be tBu, 2-methylphenyl, 2,4,6-trimethylphenyl.[2]
-
Diphosphonic acid
-
Tautomers of H3PO3:
phosphonic acid (left)
phosphorous acid (right) -
The general structure of an organic phosphonic acid
References
- ↑ Bogdán Cs, Péczely G, Hägele G (2007). "Metal Complexes of Ethane and Propane Frame-Substituted Oligophosphonic and Oligophosphonocarboxylic Acids". Phosphorus, Sulfur, and Silicon and the Related Elements. 182 (10): 2337–50. Unknown parameter
|month=ignored (help) - ↑ Diemert K, Kuchen W, Poll W, Sandt F (1998). "A Convenient Synthesis of Phosphonic Anhydrides - Trimers [RPO2]3 (R = tert-Butyl, 2-Methylphenyl, 2,4,6-Trimethylphenyl): Their Structures and Reaction Products". Eur J Inorgan Chem. 1998 (3): 361–6. doi:10.1002/(SICI)1099-0682(199803)1998:3<361::AID-EJIC361>3.0.CO;2-T.
See also
External links
- Phosphonic+Acid at the US National Library of Medicine Medical Subject Headings (MeSH)
- PubChem