Patisiran
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Patisiran is a transthyretin-directed small interfering RNA that is FDA approved for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. Common adverse reactions include upper respiratory tract infections and infusion-related reactions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Patisiran is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults.
Dosage
- For patients weighing less than 100 kg, the recommended dosage is 0.3 mg/kg every 3 weeks by intravenous infusion. For patients weighing 100 kg or more, the recommended dosage is 30 mg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding patisiran Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding patisiran Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding patisiran Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding patisiran Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
Infusion-Related Reactions
- Infusion-related reactions (IRRs) have been observed in patients treated with patisiran. In clinical studies, all patients received premedication with a corticosteroid, acetaminophen, and antihistamines (H1 and H2 blockers) to reduce the risk of IRRs. In a controlled clinical study, 19% of patisiran-treated patients experienced IRRs, compared to 9% of placebo-treated patients. Among patisiran-treated patients who experienced an IRR, 79% experienced the first IRR within the first 2 infusions. The frequency of IRRs decreased over time. IRRs led to infusion interruption in 5% of patients. IRRs resulted in permanent discontinuation of patisiran in less than 1% of patients in clinical studies. Across clinical studies, the most common symptoms (reported in greater than 2% of patients) of IRRs with patisiran were flushing, back pain, nausea, abdominal pain, dyspnea, and headache. One patient in the patisiran expanded access program had a severe adverse reaction of hypotension and syncope during a patisiran infusion.
- Patients should receive premedications on the day of patisiran infusion, at least 60 minutes prior to the start of infusion. Monitor patients during the infusion for signs and symptoms of IRRs. If an IRR occurs, consider slowing or interrupting the patisiran infusion and instituting medical management (e.g., corticosteroids or other symptomatic treatment), as clinically indicated. If the infusion is interrupted, consider resuming at a slower infusion rate only if symptoms have resolved. In the case of a serious or life-threatening IRR, the infusion should be discontinued and not resumed.
- Some patients who experience IRRs may benefit from a slower infusion rate or additional or higher doses of one or more of the premedications with subsequent infusions to reduce the risk of IRRs.
Reduced Serum Vitamin A Levels and Recommended Supplementation
- Patisiran treatment leads to a decrease in serum vitamin A levels. Supplementation at the recommended daily allowance of vitamin A is advised for patients taking patisiran. Higher doses than the recommended daily allowance of vitamin A should not be given to try to achieve normal serum vitamin A levels during treatment with patisiran, as serum vitamin A levels do not reflect the total vitamin A in the body.
- Patients should be referred to an ophthalmologist if they develop ocular symptoms suggestive of vitamin A deficiency (e.g., night blindness).
Adverse Reactions
Clinical Trials Experience
- Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of patisiran cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
- A total of 224 patients with polyneuropathy caused by hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis) received patisiran in the placebo-controlled and open-label clinical studies, including 186 patients exposed for at least 1 year, 137 patients exposed for at least 2 years, and 52 patients exposed for at least 3 years. In the placebo-controlled study, 148 patients received patisiran for up to 18 months (mean exposure 17.7 months). Baseline demographic and disease characteristics were generally similar between treatment groups. The median age of study patients was 62 years and 74% were male. Seventy-two percent of study patients were Caucasian, 23% were Asian, 2% were Black, and 2% were reported as other. At baseline, 46% of patients were in Stage 1 of the disease and 53% were in Stage 2. Forty-three percent of patients had Val30Met mutations in the transthyretin gene; the remaining patients had 38 other point mutations. Sixty-two percent of patisiran-treated patients had non-Val30Met mutations, compared to 48% of the placebo-treated patients.
- Upper respiratory tract infections and infusion-related reactions were the most common adverse reactions. One patient (0.7%) discontinued patisiran because of an infusion-related reaction.
- Table 1 lists the adverse reactions that occurred in at least 5% of patients in the patisiran-treated group and that occurred at least 3% more frequently than in the placebo-treated group in the randomized controlled clinical trial.

- Four serious adverse reactions of atrioventricular (AV) heart block (2.7%) occurred in patisiran-treated patients, including 3 cases of complete AV block. No serious adverse reactions of AV block were reported in placebo-treated patients.
- Ocular adverse reactions that occurred in 5% or less of patisiran-treated patients in the controlled clinical trial, but in at least 2% of patisiran-treated patients, and more frequently than on placebo, include dry eye (5% vs. 3%), blurred vision (3% vs. 1%), and vitreous floaters (2% vs. 1%).
- Extravasation was observed in less than 0.5% of infusions in clinical studies, including cases that were reported as serious. Signs and symptoms included phlebitis or thrombophlebitis, infusion or injection site swelling, dermatitis (subcutaneous inflammation), cellulitis, erythema or injection site redness, burning sensation, or injection site pain.
Immunogenicity
- The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. In addition, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to patisiran in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
- Anti-drug antibodies to patisiran were evaluated by measuring antibodies specific to PEG2000-C-DMG, a lipid component exposed on the surface of patisiran. In the placebo-controlled and open-label clinical studies, 7 of 194 (3.6%) patients with hATTR amyloidosis developed anti-drug antibodies during treatment with patisiran. One additional patient had pre-existing anti-drug antibodies. There was no evidence of an effect of anti-drug antibodies on clinical efficacy, safety, or the pharmacokinetic or pharmacodynamic profiles of patisiran. Although these data do not demonstrate an impact of anti-drug antibody development on the efficacy or safety of patisiran in these patients, the available data are too limited to make definitive conclusions.
Postmarketing Experience
There is limited information regarding Patisiran Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Patisiran Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no available data on patisiran use in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Patisiran treatment leads to a decrease in serum vitamin A levels, and vitamin A supplementation is advised for patients taking patisiran. Vitamin A is essential for normal embryofetal development; however, excessive levels of vitamin A are associated with adverse developmental effects. The effects on the fetus of a reduction in maternal serum TTR caused by patisiran and of vitamin A supplementation are unknown.
- In animal studies, intravenous administration of patisiran lipid complex (patisiran-LC) to pregnant rabbits resulted in developmental toxicity (embryofetal mortality and reduced fetal body weight) at doses that were also associated with maternal toxicity. No adverse developmental effects were observed when patisiran-LC or a rodent-specific (pharmacologically active) surrogate were administered to pregnant rats.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Animal Data
- Intravenous administration of patisiran-LC (0, 0.15, 0.50, or 1.5 mg/kg) or a rodent-specific (pharmacologically active) surrogate (1.5 mg/kg) to female rats every week for two weeks prior to mating and continuing throughout organogenesis resulted in no adverse effects on fertility or embryofetal development.
- Intravenous administration of patisiran-LC (0, 0.1, 0.3, or 0.6 mg/kg) to pregnant rabbits every week during the period of organogenesis produced no adverse effects on embryofetal development. In a separate study, patisiran-LC (0, 0.3, 1, or 2 mg/kg), administered to pregnant rabbits every week during the period of organogenesis, resulted in embryofetal mortality and reduced fetal body weight at the mid and high doses, which were associated with maternal toxicity.
- Intravenous administration of patisiran-LC (0, 0.15, 0.50, or 1.5 mg/kg) or a rodent-specific surrogate (1.5 mg/kg) to pregnant rats every week throughout pregnancy and lactation resulted in no adverse developmental effects on the offspring.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Patisiran in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Patisiran during labor and delivery.
Nursing Mothers
Risk Summary
- There is no information regarding the presence of patisiran in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for patisiran and any potential adverse effects on the breastfed infant from patisiran or from the underlying maternal condition.
- In lactating rats, patisiran was not detected in milk; however, the lipid components (DLin-MC3-DMA and PEG2000-C-DMG) were present in milk.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- No dose adjustment is required in patients ≥65 years old. A total of 62 patients ≥65 years of age, including 9 patients ≥75 years of age, received patisiran in the placebo-controlled study. No overall differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Patisiran with respect to specific gender populations.
Race
There is no FDA guidance on the use of Patisiran with respect to specific racial populations.
Renal Impairment
- No dose adjustment is necessary in patients with mild or moderate renal impairment (estimated glomerular filtration rate [eGFR] ≥30 to <90 mL/min/1.73m2). Patisiran has not been studied in patients with severe renal impairment or end-stage renal disease.
Hepatic Impairment
- No dose adjustment is necessary in patients with mild hepatic impairment (bilirubin ≤1 × ULN and AST >1 × ULN, or bilirubin >1.0 to 1.5 × ULN). Patisiran has not been studied in patients with moderate or severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Patisiran in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Patisiran in patients who are immunocompromised.
Administration and Monitoring
Administration
Dosing Information
- Patisiran should be administered by a healthcare professional.
- Patisiran is administered via intravenous (IV) infusion. Dosing is based on actual body weight.
- For patients weighing less than 100 kg, the recommended dosage is 0.3 mg/kg once every 3 weeks.
- For patients weighing 100 kg or more, the recommended dosage is 30 mg once every 3 weeks.
Missed Dose
- If a dose is missed, administer patisiran as soon as possible.
- If patisiran is administered within 3 days of the missed dose, continue dosing according to the patient's original schedule.
- If patisiran is administered more than 3 days after the missed dose, continue dosing every 3 weeks thereafter.
Required Premedication
- All patients should receive premedication prior to patisiran administration to reduce the risk of infusion-related reactions (IRRs). Each of the following premedications should be given on the day of patisiran infusion at least 60 minutes prior to the start of infusion:
- Intravenous corticosteroid (e.g., dexamethasone 10 mg, or equivalent)
- Oral acetaminophen (500 mg)
- Intravenous H1 blocker (e.g., diphenhydramine 50 mg, or equivalent)
- Intravenous H2 blocker (e.g., ranitidine 50 mg, or equivalent)
- For premedications not available or not tolerated intravenously, equivalents may be administered orally.
- For patients who are tolerating their patisiran infusions but experiencing adverse reactions related to the corticosteroid premedication, the corticosteroid may be reduced by 2.5 mg increments to a minimum dose of 5 mg of dexamethasone (intravenous), or equivalent.
- Some patients may require additional or higher doses of one or more of the premedications to reduce the risk of IRRs.
Preparation Instructions
- Patisiran must be filtered and diluted prior to intravenous infusion. The diluted solution for infusion should be prepared by a healthcare professional using aseptic technique as follows:
- Remove patisiran from the refrigerator and allow to warm to room temperature. Do not shake or vortex.
- Inspect visually for particulate matter and discoloration. Do not use if discoloration or foreign particles are present. Patisiran is a white to off-white, opalescent, homogeneous solution. A white to off-white coating may be observed on the inner surface of the vial, typically at the liquid-headspace interface. Product quality is not impacted by presence of the white to off-white coating.
- Calculate the required dose of patisiran based on the recommended weight-based dosage.
- Withdraw the entire contents of one or more vials into a single sterile syringe.
- Filter patisiran through a sterile 0.45 micron polyethersulfone (PES) syringe filter into a sterile container.
- Withdraw the required volume of filtered patisiran from the sterile container using a sterile syringe.
- Dilute the required volume of filtered patisiran into an infusion bag containing 0.9% Sodium Chloride Injection, USP for a total volume of 200 mL. Use infusion bags that are di(2-ethylhexyl)phthalate-free (DEHP-free).
- Gently invert the bag to mix the solution. Do not shake. Do not mix or dilute with other drugs.
- Discard any unused portion of patisiran.
- Patisiran does not contain preservatives. The diluted solution should be administered immediately after preparation. If not used immediately, store in the infusion bag at room temperature (up to 30°C [86°F]) for up to 16 hours (including infusion time). Do not freeze.
Infusion Instructions
- Use a dedicated line with an infusion set containing a 1.2 micron polyethersulfone (PES) in-line infusion filter. Use infusion sets and lines that are DEHP-free.
- Infuse the diluted solution of patisiran intravenously, via an ambulatory infusion pump, over approximately 80 minutes, at an initial infusion rate of approximately 1 mL/min for the first 15 minutes, then increase to approximately 3 mL/min for the remainder of the infusion. The duration of infusion may be extended in the event of an IRR [see WARNINGS AND PRECAUTIONS].
- Administer only through a free-flowing venous access line. Monitor the infusion site for possible infiltration during drug administration. Suspected extravasation should be managed according to local standard practice for non-vesicants.
- Observe the patient during the infusion and, if clinically indicated, following the infusion.
- After completion of the infusion, flush the intravenous administration set with 0.9% Sodium Chloride Injection, USP to ensure that all patisiran has been administered.
Monitoring
There is limited information regarding Patisiran Monitoring in the drug label.
IV Compatibility
- Patisiran is administered as an intravenous injection.
Overdosage
There is limited information regarding Patisiran overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Patisiran is a double-stranded siRNA that causes degradation of mutant and wild-type TTR mRNA through RNA interference, which results in a reduction of serum TTR protein and TTR protein deposits in tissues.
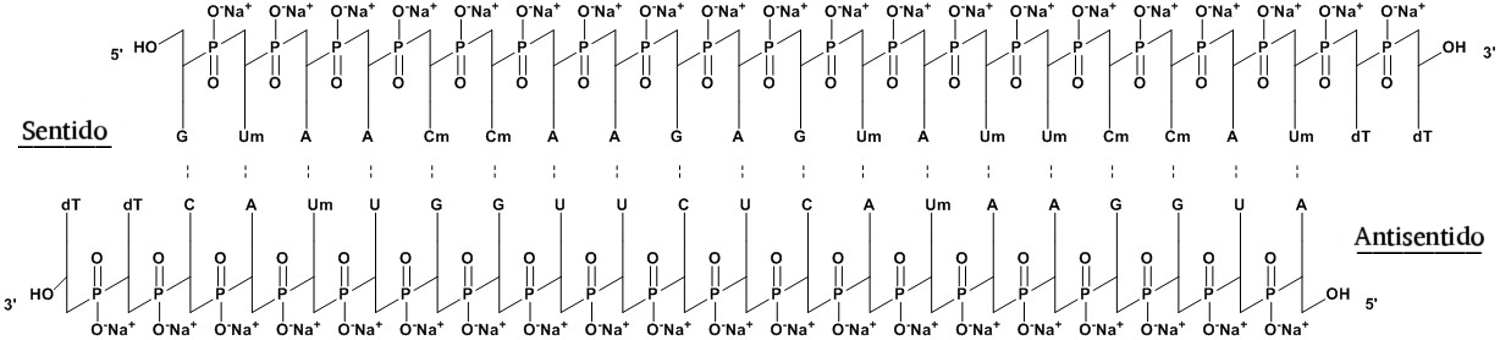
Structure
- Onpattro contains patisiran, a double-stranded small interfering ribonucleic acid (siRNA), formulated as a lipid complex for delivery to hepatocytes. Patisiran specifically binds to a genetically conserved sequence in the 3' untranslated region (3'UTR) of mutant and wild-type transthyretin (TTR) messenger RNA (mRNA).
- The molecular formula of patisiran sodium is C412H480N148Na40O290P40 and the molecular weight is 14304 Da.
- The structural formula is:

Pharmacodynamics
- The pharmacodynamic effects of patisiran were evaluated in hATTR amyloidosis patients treated with 0.3 mg/kg patisiran via intravenous infusion once every 3 weeks.
- Mean serum TTR was reduced by approximately 80% within 10 to 14 days after a single dose. With repeat dosing every 3 weeks, mean reductions of serum TTR after 9 and 18 months of treatment were 83% and 84%, respectively. The mean maximum reduction of serum TTR over 18 months was 88%. Similar TTR reductions were observed regardless of TTR mutation, sex, age, or race. In a dose-ranging study, greater TTR reduction was maintained over the dosing interval with the recommended dosing regimen of 0.3 mg/kg every 3 weeks compared to 0.3 mg/kg every 4 weeks.
- Serum TTR is a carrier of retinol binding protein, which is involved in the transport of vitamin A in the blood. Mean reductions in serum retinol binding protein of 45% and serum vitamin A of 62% were observed over 18 months.
Pharmacokinetics
- Following a single intravenous administration, systemic exposure to patisiran increases in a linear and dose-proportional manner over the range of 0.01 to 0.5 mg/kg. Greater than 95% of patisiran in the circulation is associated with the lipid complex. At the recommended dosing regimen of 0.3 mg/kg every 3 weeks, steady state is reached by 24 weeks of treatment. The estimated mean ± SD steady state peak concentrations (Cmax), trough concentrations (Ctrough), and area under the curve (AUCτ) were 7.15 ± 2.14 µg/mL, 0.021 ± 0.044 µg/mL, and 184 ± 159 µg∙h/mL, respectively. The accumulation of AUCτ was 3.2-fold at steady state, compared to the first dose. In the placebo-controlled study, inter-patient variability in patisiran exposure did not result in differences in clinical efficacy (mNIS+7 change from baseline) or safety (adverse events, serious adverse events).
Distribution
- Plasma protein binding of patisiran is low, with ≤2.1% binding observed in vitro with human serum albumin and human α1-acid glycoprotein. Patisiran distributes primarily to the liver. At the recommended dosing regimen of 0.3 mg/kg every 3 weeks, the mean ± SD steady state volume of distribution of patisiran (Vss) was 0.26 ± 0.20 L/kg.
Elimination
- The terminal elimination half-life (mean ± SD) of patisiran is 3.2 ± 1.8 days. Patisiran is mainly cleared through metabolism, and the total body clearance (mean ± SD) at steady state (CLss) is 3.0 ± 2.5 mL/h/kg.
Metabolism
- Patisiran is metabolized by nucleases to nucleotides of various lengths.
Excretion
- Less than 1% of the administered dose of patisiran is excreted unchanged into urine.
Specific Populations
- Age, race (non-Caucasian vs. Caucasian), and sex had no impact on the steady state pharmacokinetics of patisiran or TTR reduction. Population pharmacokinetic and pharmacodynamic analyses indicated no impact of mild or moderate renal impairment (eGFR ≥30 to <90 mL/min/1.73m2) or mild hepatic impairment (bilirubin ≤1 × ULN and AST >1 × ULN, or bilirubin >1.0 to 1.5 × ULN) on patisiran exposure or TTR reduction. Patisiran has not been studied in patients with severe renal impairment, end-stage renal disease, moderate or severe hepatic impairment, or in patients with prior liver transplant.
Drug Interaction Studies
- No formal clinical drug interaction studies have been performed. The components of patisiran are not inhibitors or inducers of cytochrome P450 enzymes or transporters. Patisiran is not a substrate of cytochrome P450 enzymes. In a population pharmacokinetic analysis, concomitant use of strong or moderate CYP3A inducers and inhibitors did not impact the pharmacokinetic parameters of patisiran. Patisiran is not expected to cause drug-drug interactions or to be affected by inhibitors or inducers of cytochrome P450 enzymes.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Patisiran-LC was not carcinogenic in TgRasH2 mice when administered at intravenous (IV) doses of 0, 0.5, 2, or 6 mg/kg every two weeks for 26 weeks.
Mutagenesis
- Patisiran-LC was negative for genotoxicity in in vitro (bacterial mutagenicity assay, chromosomal aberration assay in human peripheral blood lymphocytes) and in vivo (mouse bone marrow micronucleus) assays.
Impairment of Fertility
- Intravenous (IV) administration of patisiran-LC (0, 0.03, 0.1, or 0.3 mg/kg) or a rodent-specific (pharmacologically active) surrogate (0.1 mg/kg) to male rats every two weeks prior to and throughout mating to untreated females produced no adverse effects on fertility.
- Intravenous administration of patisiran-LC (0, 0.15, 0.50, or 1.5 mg/kg) or a rodent-specific (pharmacologically active) surrogate (1.5 mg/kg) to female rats every week for two weeks prior to mating and continuing throughout organogenesis resulted in no adverse effects on fertility or on embryofetal development.
- Intravenous administration of patisiran-LC (0, 0.3, 1, or 2 mg/kg) to adult monkeys every three weeks for 39 weeks produced no adverse effects on male reproductive organs or on sperm morphology or count.
Clinical Studies
- The efficacy of patisiran was demonstrated in a randomized, double-blind, placebo-controlled, multicenter clinical trial in adult patients with polyneuropathy caused by hATTR amyloidosis (NCT 01960348). Patients were randomized in a 2:1 ratio to receive patisiran 0.3 mg/kg (N=148) or placebo (N=77), respectively, via intravenous infusion once every 3 weeks for 18 months. All patients received premedication with a corticosteroid, acetaminophen, and H1 and H2 blockers. Ninety-three percent of patisiran-treated patients and 62% of placebo-treated patients completed 18 months of the assigned treatment.
- The primary efficacy endpoint was the change from baseline to Month 18 in the modified Neuropathy Impairment Score +7 (mNIS+7). The mNIS+7 is an objective assessment of neuropathy and comprises the NIS and Modified +7 (+7) composite scores. In the version of the mNIS+7 used in the trial, the NIS objectively measures deficits in cranial nerve function, muscle strength, and reflexes, and the +7 assesses postural blood pressure, quantitative sensory testing, and peripheral nerve electrophysiology. The maximum possible score was 304 points, with higher scores representing a greater severity of disease.
- The clinical meaningfulness of effects on the mNIS+7 was assessed by the change from baseline to Month 18 in Norfolk Quality of Life-Diabetic Neuropathy (QoL-DN) total score. The Norfolk QoL-DN scale is a patient-reported assessment that evaluates the subjective experience of neuropathy in the following domains: physical functioning/large fiber neuropathy, activities of daily living, symptoms, small fiber neuropathy, and autonomic neuropathy. The version of the Norfolk QoL-DN that was used in the trial had a total score range from -4 to 136, with higher scores representing greater impairment.
- The changes from baseline to Month 18 on both the mNIS+7 and the Norfolk QoL-DN significantly favored patisiran (Table 2, Figure 1 and Figure 3). The distributions of changes in mNIS+7 and Norfolk QoL-DN scores from baseline to Month 18 by percent of patients are shown in Figure 2 and Figure 4, respectively.
- The changes from baseline to Month 18 in modified body mass index (mBMI) and gait speed (10-meter walk test) significantly favored patisiran (Table 2).


- A decrease in mNIS+7 indicates improvement.
- Δ indicates between-group treatment difference, shown as the LS mean difference (95% CI) for patisiran – placebo.

- mNIS+7 change scores are rounded to the nearest whole number; last available post-baseline scores were used.
- Categories are mutually exclusive; patients who died before 18 months are summarized in the "Death" category only.

- A decrease in Norfolk QoL-DN score indicates improvement.
- Δ indicates between-group treatment difference, shown as the LS mean difference (95% CI) for patisiran – placebo.

- Norfolk QoL-DN change scores are rounded to the nearest whole number; last available post-baseline scores were used.
- Categories are mutually exclusive; patients who died before 18 months are summarized in the "Death" category only.
- Patients receiving patisiran experienced similar improvements relative to placebo in mNIS+7 and Norfolk QoL-DN score across all subgroups including age, sex, race, region, NIS score, Val30Met mutation status, and disease stage.
How Supplied
- Patisiran is a sterile, preservative-free, white to off-white, opalescent, homogeneous solution for intravenous infusion supplied as a 10 mg/5 mL (2 mg/mL) solution in a single-dose glass vial. The vial stopper is not made with natural rubber latex. Patisiran is available in cartons containing one single-dose vial each.
Storage
- Store at 2°C to 8°C (36°F to 46°F). Do not freeze. Discard vial if it has been frozen.
- If refrigeration is not available, patisiran can be stored at room temperature up to 25°C (up to 77°F) for up to 14 days.
- For storage conditions of patisiran after dilution in the infusion bag, see Administration & Monitoring
Images
Drug Images
{{#ask: Page Name::Patisiran |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Patisiran |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Infusion-Related Reactions
- Inform patients about the signs and symptoms of infusion-related reactions (e.g., flushing, dyspnea, chest pain, rash, increased heart rate, facial edema). Advise patients to contact their healthcare provider immediately if they experience signs and symptoms of infusion-related reactions.
Recommended Vitamin A Supplementation
- Inform patients that patisiran treatment leads to a decrease in vitamin A levels measured in the serum. Instruct patients to take the recommended daily allowance of vitamin A. Advise patients to contact their healthcare provider if they experience ocular symptoms suggestive of vitamin A deficiency (e.g., night blindness) and refer them to an ophthalmologist if they develop these symptoms.
Pregnancy
- Instruct patients that if they are pregnant or plan to become pregnant while taking patisiran they should inform their healthcare provider. Advise female patients of childbearing potential of the potential risk to the fetus.
Precautions with Alcohol
Alcohol-Patisiran interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Patisiran Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.