Oxacillin sodium dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
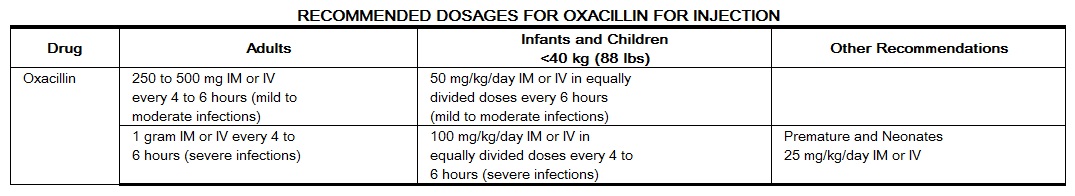
Dosage and Administration
The penicillinase-resistant penicillins are available for oral administration and for intramuscular and intravenous injection. The sodium salts of methicillin, oxacillin, and nafcillin may be administered parenterally and the sodium salts of cloxacillin, dicloxacillin, oxacillin, and nafcillin are available for oral use.
Bacteriologic studies to determine the causative organisms and their susceptibility to oxacillin should always be performed. Duration of therapy varies with the type of severity of infection as well as the overall condition of the patient; therefore, it should be determined by the clinical and bacteriological response of the patient. In severe staphylococcal infections, therapy with oxacillin should be continued for at least 14 days. Therapy should be continued for at least 48 hours after the patient has become afebrile, asymptomatic, and cultures are negative. Treatment of endocarditis and osteomyelitis may require a longer duration of therapy.
Concurrent administration of oxacillin and probenecid increases and prolongs serum penicillin levels. Probenecid decreases the apparent volume of distribution and slows the rate of excretion by competitively inhibiting renal tubular secretion of penicillin. Penicillin-probenecid therapy is generally limited to those infections where very high serum levels of penicillin are necessary.
Oral preparations of the penicillinase-resistant penicillins should not be used as initial therapy in serious, life-threatening infections (see PRECAUTIONS-General). Oral therapy with the penicillinase-resistant penicillins may be used to follow-up the previous use of a parenteral agent as soon as the clinical condition warrants. For intramuscular gluteal injections, care should be taken to avoid sciatic nerve injury. With intravenous administration, particularly in elderly patients, care should be taken because of the possibility of thrombophlebitis.
|
Directions for Use
- For Intramuscular Use
Use Sterile Water for Injection, USP. Add 5.7 mL to the 1 gram vial and 11.5 mL to the 2 gram vial. Shake well until a clear solution is obtained. After reconstitution, vials will contain 250 mg of active drug per 1.5 mL of solution. The reconstituted solution is stable for 3 days at 70°F or for one week under refrigeration (40°F).
- For Direct Intravenous Use
Use Sterile Water for Injection, USP or Sodium Chloride Injection, USP. Add 10 mL to the 1 gram vial and 20 mL to the 2 gram vial. Withdraw the entire contents and administer slowly over a period of approximately 10 minutes.
- For Administration by Intravenous Drip
Reconstitute as directed above (For Direct Intravenous Use) prior to diluting with Intravenous Solution.
|
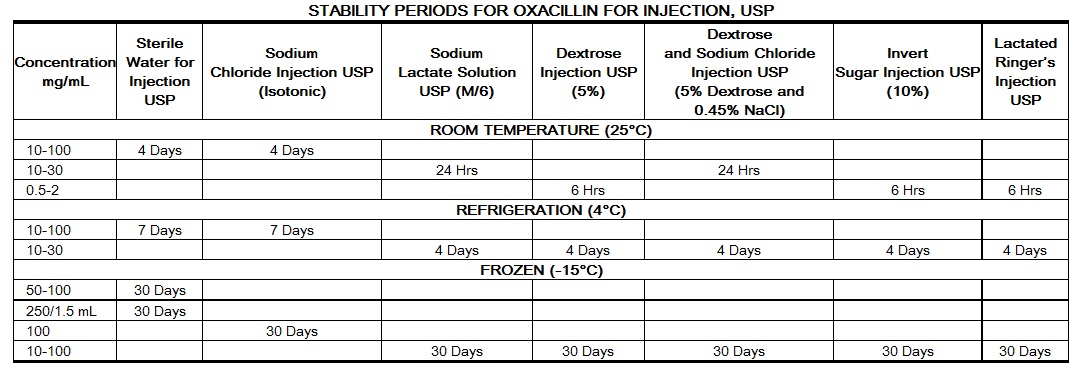
Stability studies on oxacillin sodium at concentrations of 0.5 mg/mL and 2 mg/mL in various intravenous solutions listed below indicate the drug will lose less than 10% activity at room temperature (70°F) during a 6-hour period.
IV Solution
- 5% Dextrose in Normal Saline
- 10% D-Fructose in Water
- 10% D-Fructose in Normal Saline
- Lactated Potassic Saline Injection
- 10% Invert Sugar in Normal Saline
- 10% Invert Sugar Plus 0.3% Potassium Chloride in Water
- Travert 10% Electrolyte #1
- Travert 10% Electrolyte #2
- Travert 10% Electrolyte #3
Only those solutions listed above should be used for the intravenous infusion of oxacillin sodium. The concentration of the antibiotic should fall within the range specified. The drug concentration and the rate and volume of the infusion should be adjusted so that the total dose of oxacillin is administered before the drug loses its stability in the solution in use.
If another agent is used in conjunction with oxacillin therapy, it should not be physically mixed with oxacillin but should be administered separately.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Do not add supplementary medication to Oxacillin for Injection, USP.[1]
References
- ↑ "OXACILLIN (OXACILLIN SODIUM) INJECTION, POWDER, FOR SOLUTION [AUROMEDICS PHARMA LLC]". Text " accessdate" ignored (help)
Adapted from the FDA Package Insert.