Steroid
|
WikiDoc Resources for Steroid |
|
Articles |
|---|
|
Most recent articles on Steroid |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Steroid at Clinical Trials.gov Clinical Trials on Steroid at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Steroid
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Steroid Risk calculators and risk factors for Steroid
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Steroid |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
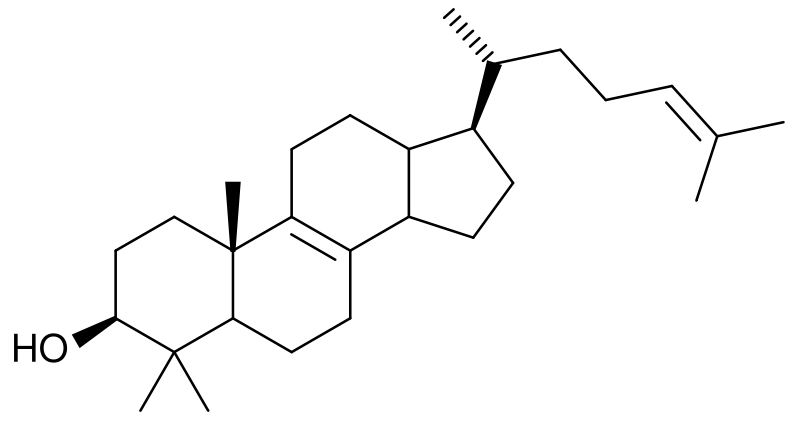
A steroid is a terpenoid lipid characterized by a carbon skeleton with four fused rings, generally arranged in a 6-6-6-5 fashion.
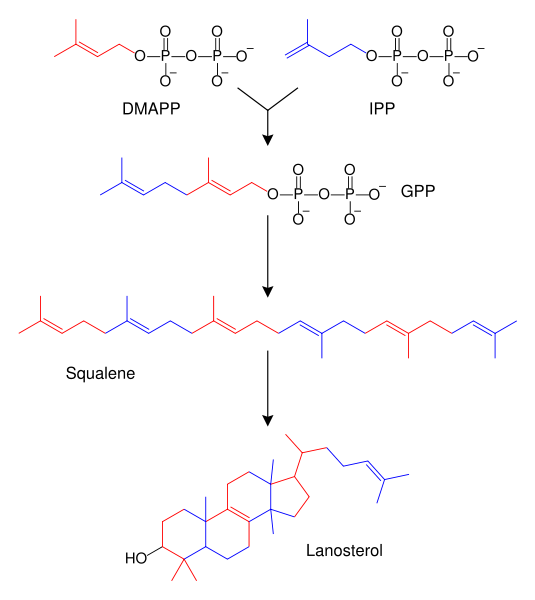
Steroids can vary by the functional groups attached to these rings and the oxidation state of the rings. Hundreds of distinct steroids are found in plants, animals, and fungi. All steroids are biosynthetically derived either from the sterol lanosterol (animals and fungi) or the sterol cycloartenol (plants). Both sterols are derived from the cyclization of the triterpene squalene.[1]
Origin
Steroids include estrogen (US spelling) or oestrogen (UK/AUS spelling), progesterone and testosterone. Oestrogen and progesterone are made primarily in the ovary and in the placenta during pregnancy and testosterone in the testes. Certain neurons and glia in the central nervous system (CNS) express the enzymes that are required for the local synthesis of pregnane neurosteroids, either de novo or from peripherally derived sources.
Classification
Taxonomical/Functional
Some of the common categories of steroids:
- Animal steroids
- Insect steroids
- Ecdysteroids such as ecdysterone
- Vertebrate steroids
- Steroid hormones
- Sex steroids are a subset of sex hormones that produce sex differences or support reproduction. They include androgens, estrogens, and progestagens.
- Corticosteroids include glucocorticoids and mineralocorticoids. Glucocorticoids regulate many aspects of metabolism and immune function, whereas mineralocorticoids help maintain blood volume and control renal excretion of electrolytes.
- Anabolic steroids are a class of steroids that interact with androgen receptors to increase muscle and bone synthesis. There are natural and synthetic anabolic steroids. In popular language the word "steroids" usually refers to anabolic steroids.
- Cholesterol which modulates the fluidity of cell membranes and is the principle constituent of the plaques implicated in atherosclerosis.
- Steroid hormones
- Insect steroids
- Plant steroids
- Fungus steroids
Structural
It is also possible to classify steroids based upon their chemical composition.
See Also
References
bg:Стероид ca:Esteroide cs:Steroidy de:Steroide eo:Steroido ko:스테로이드 it:Steroide he:סטרואיד nl:Steroïde no:Steroid simple:Steroid sk:Steroid sr:Стероиди fi:Steroidi sv:Steroid th:สเตอรอยด์