Natamycin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Natamycin is an antifungal polyene that is FDA approved for the treatment of fungal blepharitis, conjunctivitis, and keratitis caused by susceptible organisms including Fusarium solani keratitis. Common adverse reactions include Eye irritation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Fungal Keratitis, Blepharitis and Conjunctivitis
- One drop natamycin ophthalmic suspension 5% instilled in the conjunctival sac at hourly or two-hourly intervals.
- The frequency of application can usually be reduced to one drop 6 to 8 times daily after the first 3 to 4 days.
- Therapy should generally be continued for 14 to 21 days or until there is resolution of active fungal keratitis. In many cases, it may be helpful to reduce the dosage gradually at 4 to 7 day intervals to assure that the replicating organism has been eliminated.
- Less frequent initial dosage (4 to 6 daily applications) may be sufficient in fungal blepharitis and conjunctivitis.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Natamycin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Natamycin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Natamycin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Natamycin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Natamycin in pediatric patients.
Contraindications
- Contraindicated in individuals with a history of hypersensitivity to any of its components.
Warnings
- FOR TOPICAL OPHTHALMIC USE ONLY — NOT FOR INJECTION.
- Failure of improvement of keratitis following 7-10 days of administration of the drug suggests that the infection may be caused by a microorganism not susceptible to natamycin.
- Continuation of therapy should be based on clinical re-evaluation and additional laboratory studies.
- Adherence of the suspension to areas of epithelial ulceration or retention of the suspension in the fornices occurs regularly.
- Use only if the container is undamaged.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Natamycin Clinical Trials Experience in the drug label.
Postmarketing Experience
- The following events have been identified during post-marketing use of natamycin in clinical practice.
- Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made.
- The events, which have been chosen for inclusion due to their seriousness, frequency of reporting, possible causal connection to natamycin, or a combination of these factors include:
- Allergic reaction
- Change in vision,
- Chest pain
- Corneal opacity
- Dyspnea
- Eye discomfort
- Eye edema
- Eye hyperemia
- Eye irritation
- Eye pain
- Foreign body sensation
- Parethesia
- Tearing
Drug Interactions
There is limited information regarding Natamycin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Animal reproduction studies have not been conducted with natamycin. It is also not known whether natamycin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Natamycin (natamycin ophthalmic suspension) 5% should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Natamycin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Natamycin during labor and delivery.
Nursing Mothers
It is not known whether these drugs are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when natamycin is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Natamycin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Natamycin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Natamycin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Natamycin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Natamycin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Natamycin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic
Monitoring
There is limited information regarding Natamycin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Natamycin and IV administrations.
Overdosage
There is limited information regarding Natamycin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Template:Chembox E numberTemplate:Chembox AppearanceTemplate:Chembox DensityTemplate:Chembox MeltingPtTemplate:Chembox SolubilityInWater
| |
| Names | |
|---|---|
| IUPAC name
(1R,3S,5R,7R,8E,12R,14E,16E,18E,20E,22R,24S,25R,26S)-22-[(3-amino-3,6-dideoxy-D-mannopyranosyl)oxy]-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C33H47NO13 | |
| Molar mass | 665.725 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mechanism of Action
Natamycin is a tetraene polyene antibiotic derived from Streptomyces natalensis. It possesses in vitro activity against a variety of yeast and filamentous fungi, including Candida, Aspergillus, Cephalosporium, Fusarium and Penicillium. The mechanism of action appears to be through binding of the molecule to the sterol moiety of the fungal cell membrane. The polyenesterol complex alters the permeability of the membrane to produce depletion of essential cellular constituents. Although the activity against fungi is dose-related, natamycin is predominantly fungicidal. Natamycin is not effective in vitro against gram-positive or gram-negative bacteria.
Structure
- Natamycin ophthalmic suspension) 5% is a sterile, antifungal drug for topical ophthalmic administration.
- Each mL of the suspension contains:
- Active: natamycin 5% (50 mg).
- Preservative: benzalkonium chloride 0.02%.
- Inactive: sodium hydroxide and/or hydrochloric acid (neutralized to adjust the pH), purified water.
- The active ingredient is represented by the chemical structure:
- Established name: Natamycin
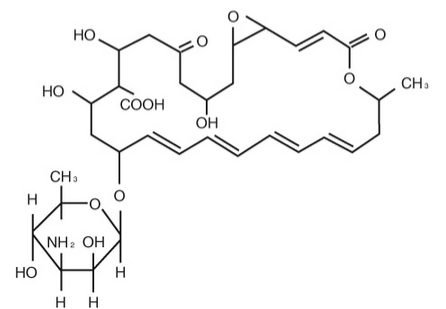
- Molecular Formula: C33H47NO13
- Molecular Weight: 665.73
- Chemical name: Stereoisomer of 22-[(3-amino-3,6-dideoxy- β-D-mannopyranosyl)oxy]-1,3,26- trihydroxy-12- methyl-10-oxo-6,11,28- trioxatricyclo[22.3.1.05,7] octacosa-8,14,16,18,20-pentaene-25- carboxylic acid.
- Other: Pimaricin
- The pH range is 5.0 - 7.5.
Pharmacodynamics
There is limited information regarding Natamycin Pharmacodynamics in the drug label.
Pharmacokinetics
Topical administration appears to produce effective concentrations of natamycin within the corneal stroma but not in intraocular fluid. Systemic absorption should not be expected following topical administration of natamycin (natamycin ophthalmic suspension) 5%. As with other polyene antibiotics, absorption from the gastrointestinal tract is very poor. Studies in rabbits receiving topical natamycin revealed no measurable compound in the aqueous humor or sera, but the sensitivity of the measurement was no greater than 2 mg/mL.
Nonclinical Toxicology
There have been no long term studies done using natamycin in animals to evaluate carcinogenesis, mutagenesis, or impairment of fertility.
Clinical Studies
There is limited information regarding Natamycin Clinical Studies in the drug label.
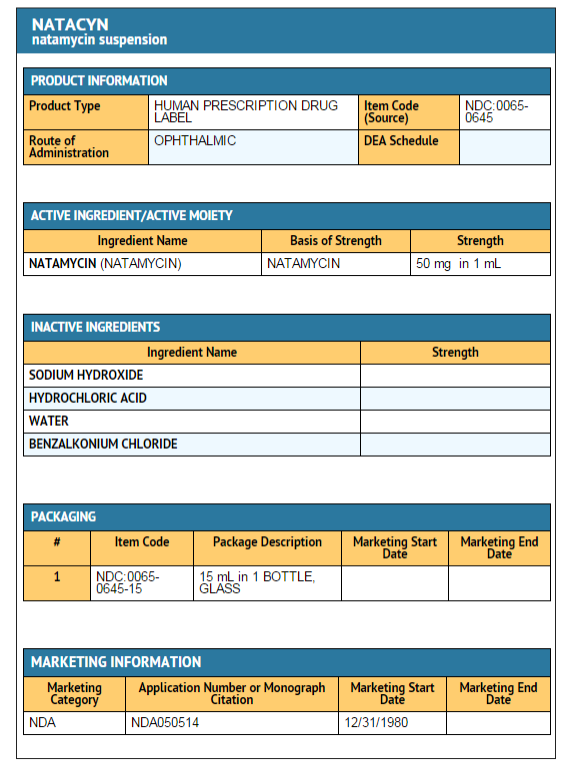
How Supplied
- Natamycin ophthalmic suspension 5% is a 15mL fill packaged in a 15mL amber glass bottle with a black phenolic closure.
- A flint glass dropper with a red plastic closure and a black rubber bulb are packaged separately in a clear plastic blister with Tyvek backing.
- NDC 0065-0645-15
Storage
- Store between 2-24°C (36-75°F).
- Do not freeze.
- Avoid exposure to light and excessive heat.
Images
Drug Images
{{#ask: Page Name::Natamycin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Natamycin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Do not touch dropper tip to any surface, as this may contaminate the suspension. *Patients should be advised not to wear contact lenses if they have signs and symptoms of fungal blepharitis, conjunctivitis, and keratitis.
Precautions with Alcohol
Alcohol-Natamycin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Natacyn[1]
Look-Alike Drug Names
There is limited information regarding Natamycin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.