Mometasone (inhalation)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Mometasone (inhalation) is a glucocorticoid and antiasthmatic agent that is FDA approved for the treatment of asthma. Common adverse reactions include nasopharyngitis, headache, sinusitis, bronchitis, and influenza.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Treatment of Asthma
- ASMANEX® HFA is indicated for the maintenance treatment of asthma as prophylactic therapy in patients 12 years of age and older.
- Important Limitations of Use
- ASMANEX HFA is NOT indicated for the relief of acute bronchospasm.
Dosing
- ASMANEX HFA should be administered as two inhalations twice daily every day (morning and evening) by the orally inhaled route.
- Shake well prior to each inhalation.
- The recommended doses for ASMANEX HFA treatment based on prior asthma therapy are provided in Table 1.
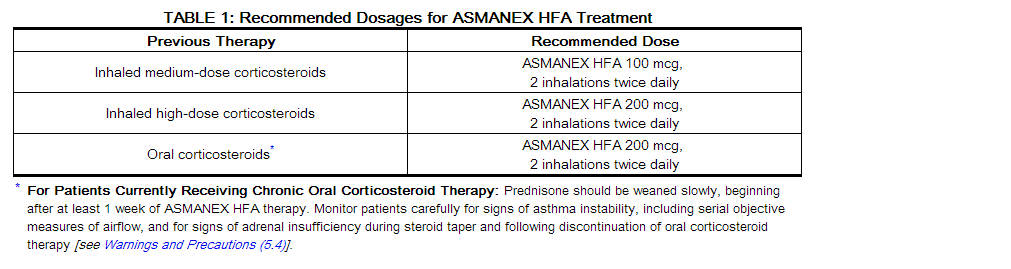
- If a dosage regimen of ASMANEX HFA fails to provide adequate control of asthma, the therapeutic regimen should be re-evaluated and additional therapeutic options, e.g., replacing the current strength of ASMANEX HFA with a higher strength, initiating an inhaled corticosteroid and long-acting beta2-agonist combination product, or initiating oral corticosteroids, should be considered.
- The maximum daily recommended dose is two inhalations of ASMANEX HFA 200 mcg twice daily (maximum of 800 mcg a day). If symptoms arise between doses, an inhaled short-acting beta2-agonist should be taken for immediate relief.
- The maximum benefit may not be achieved for 1 week or longer after beginning treatment. Individual patients may experience a variable time to onset and degree of symptom relief. For patients who do not respond adequately after 2 weeks of therapy, higher strength may provide additional asthma control.
- After asthma stability has been achieved, it is desirable to titrate to the lowest effective dosage to reduce the possibility of side effects.
DOSAGE FORMS AND STRENGTHS
- ASMANEX HFA is a pressurized metered dose inhaler that is available in 2 strengths.
- ASMANEX HFA 100 mcg delivers 100 mcg of mometasone furoate per actuation.
- ASMANEX HFA 200 mcg delivers 200 mcg of mometasone furoate per actuation
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mometasone (inhalation) in adult patients.
Non–Guideline-Supported Use
Rhinitis and Sinusitis
- Dosing Information
- 200–400 mcg[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- The safety and efficacy of ASMANEX HFA have not been established in children less than 12 years of age.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Mometasone (inhalation) in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Mometasone (inhalation) in pediatric patients.
Contraindications
Status Asthmaticus
- ASMANEX HFA is contraindicated in the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required.
Hypersensitivity
- ASMANEX HFA is contraindicated in patients with known hypersensitivity to mometasone furoate or any of the ingredients in ASMANEX HFA.
Warnings
Deterioration of Asthma and Acute Episodes
- ASMANEX HFA is not indicated for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. An inhaled, short-acting beta2-agonist, not ASMANEX HFA, should be used to relieve acute symptoms such as shortness of breath. When prescribing ASMANEX HFA, the physician must also provide the patient with an inhaled, short-acting beta2-agonist (e.g., albuterol) for treatment of acute symptoms, despite regular twice-daily (morning and evening) use of ASMANEX HFA. Instruct patients to contact their physician immediately if episodes of asthma that are not responsive to bronchodilators occur during the course of treatment with ASMANEX HFA. During such episodes, patients may require therapy with oral corticosteroids.
Local Effects
- In clinical trials, the development of localized infections of the mouth and pharynx with Candida albicans have occurred in patients treated with ASMANEX HFA. If oropharyngeal candidiasis develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while remaining on treatment with ASMANEX HFA therapy, but at times therapy with ASMANEX HFA may need to be interrupted. Advise patients to rinse the mouth after inhalation of ASMANEX HFA.
Immunosuppression
- Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals.
- Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or who are not properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
- Inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract, untreated systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
Transferring Patients from Systemic Corticosteroid Therapy
- Particular care is needed for patients who are transferred from systemically active corticosteroids to ASMANEX HFA because deaths due to adrenal insufficiency have occurred in asthmatic patients during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
- Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although ASMANEX HFA may improve control of asthma symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of corticosteroid systemically and does NOT provide the mineralocorticoid activity necessary for coping with these emergencies.
- During periods of stress or severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physicians for further instruction. These patients should also be instructed to carry a medical identification card indicating that they may need supplementary systemic corticosteroids during periods of stress or severe asthma attack.
- Patients requiring oral or other systemic corticosteroids should be weaned slowly from oral or other systemic corticosteroid use after transferring to ASMANEX HFA. Lung function (FEV1 or PEF), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral or other systemic corticosteroids. In addition to monitoring asthma signs and symptoms, patients should be observed for signs and symptoms of adrenal insufficiency such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
- Transfer of patients from systemic corticosteroid therapy to ASMANEX HFA may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy, e.g., rhinitis, conjunctivitis, eczema, arthritis, and eosinophilic conditions.
- During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal, e.g., joint and/or muscular pain, lassitude, and depression, despite maintenance or even improvement of respiratory function.
Hypercorticism and Adrenal Suppression
- ASMANEX HFA will often help control asthma symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since mometasone furoate is absorbed into the circulation and can be systemically active at higher doses, the beneficial effects of ASMANEX HFA in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose.
- Because of the possibility of systemic absorption of inhaled corticosteroids, patients treated with ASMANEX HFA should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
- It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients, particularly when mometasone furoate is administered at higher than recommended doses over prolonged periods of time. If such effects occur, the dosage of ASMANEX HFA should be reduced slowly, consistent with accepted procedures for reducing systemic corticosteroids and for management of asthma symptoms.
Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
- Caution should be exercised when considering the coadministration of ASMANEX HFA with ketoconazole, and other known strong cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4) inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) because adverse effects related to increased systemic exposure to mometasone furoate may occur.
Paradoxical Bronchospasm and Upper Airway Symptoms
- ASMANEX HFA may produce inhalation induced bronchospasm with an immediate increase in wheezing after dosing that may be life-threatening. If inhalation induced bronchospasm occurs, it should be treated immediately with an inhaled, short-acting bronchodilator. ASMANEX HFA should be discontinued immediately and alternative therapy instituted.
Hypersensitivity Reactions Including Anaphylaxis
- Hypersensitivity reactions such as urticaria, flushing, allergic dermatitis, and bronchospasm, may occur after administration of ASMANEX HFA. Discontinue ASMANEX HFA if such reactions occur .
- The following additional hypersensitivity reactions, such as rash, pruritus, angioedema, and anaphylactic reaction, have been reported after administration of mometasone furoate dry powder inhaler.
Reduction in Bone Mineral Density
- Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing inhaled corticosteroids, including mometasone furoate. The clinical significance of small changes in BMD with regard to long-term outcomes, such as fracture, is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants and corticosteroids) should be monitored and treated with established standards of care.
- In a 2-year double-blind study in 103 male and female asthma patients 18 to 50 years of age previously maintained on bronchodilator therapy (Baseline FEV1 85%-88% predicted), treatment with mometasone furoate dry powder inhaler 200 mcg twice daily resulted in significant reductions in lumbar spine (LS) BMD at the end of the treatment period compared to placebo. The mean change from Baseline to Endpoint in the lumbar spine BMD was -0.015 (-1.43%) for the mometasone furoate dry powder inhaler group compared to 0.002 (0.25%) for the placebo group. In another 2-year double-blind study in 87 male and female asthma patients 18 to 50 years of age previously maintained on bronchodilator therapy (Baseline FEV1 82%-83% predicted), treatment with mometasone furoate dry powder inhaler 400 mcg twice daily demonstrated no statistically significant changes in lumbar spine BMD at the end of the treatment period compared to placebo. The mean change from Baseline to Endpoint in the lumbar spine BMD was -0.018 (-1.57%) for the mometasone furoate group compared to -0.006 (-0.43%) for the placebo group.
Effect on Growth
- Orally inhaled corticosteroids, including ASMANEX HFA, may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth of pediatric patients receiving ASMANEX HFA routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including ASMANEX HFA, titrate each patient's dose to the lowest dosage that effectively controls his/her symptoms.
Glaucoma and Cataracts
- Glaucoma, increased intraocular pressure, and cataracts have been reported following the use of long-term administration of inhaled corticosteroids, including mometasone furoate. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of ASMANEX HFA was evaluated in 2 randomized placebo and active-controlled trials of 12 and 26 weeks' duration, conducted as part of a mometasone furoate/formoterol fumarate combination product asthma program, which enrolled 1509 patients with persistent asthma. Patient ages ranged from 12 to 84 years of age, 41% were male and 59% female, 73% were Caucasian and 27% non-Caucasian. Of the total population enrolled in the 2 trials, 432 patients received two inhalations twice daily of either ASMANEX HFA, 100 mcg or 200 mcg/actuation. In the 26-week trial (Trial 1) 192 patients received two inhalations twice daily of ASMANEX HFA 100 mcg/actuation and 196 patients received placebo. In the 12 week trial (Trial 2) 240 patients received two inhalations twice daily of ASMANEX HFA 200 mcg/actuation and 233 and 255 patients received mometasone furoate and formoterol fumarate 100 mcg/5 mcg and 200 mcg/5 mcg/actuation combination products, respectively, as comparators.
- In these trials, the proportion of patients who discontinued study treatment early due to adverse reactions was 3% and 2% for ASMANEX HFA 100 and 200 mcg treated patients, respectively, and 4% for placebo-treated patients. Serious adverse reactions, whether considered drug-related or not by the investigators, which occurred more frequently in ASMANEX HFA-treated patients included colitis ulcerative, colonic polyp, chest pain, gastroenteritis, endometriosis, asthma, and hemoptysis; all events occurred at rates less than 1%.
- The incidence of treatment emergent adverse reactions associated with ASMANEX HFA are shown in Tables 2 and 3. These are based upon data from each of the 2 clinical trials of 12 or 26 weeks in duration in patients 12 years and older treated with two inhalations twice daily of ASMANEX HFA (100 mcg or 200 mcg), mometasone furoate/formoterol fumarate (100 mcg/5 mcg or 200 mcg/5 mcg), or placebo.
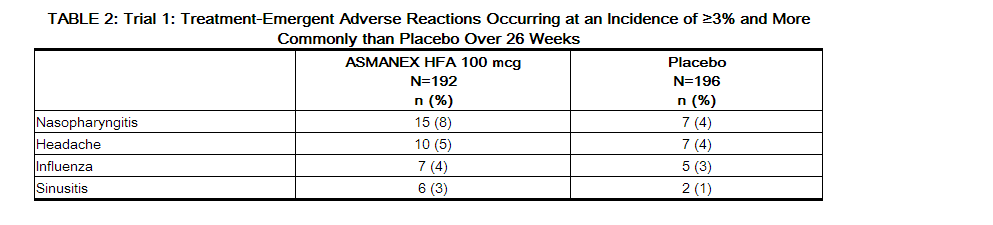
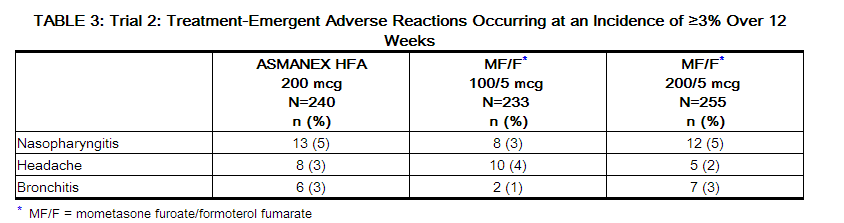
- Oral candidiasis has been reported in clinical trials at an incidence of 0.5% in patients using ASMANEX HFA 100 mcg, 0.8% in patients using ASMANEX HFA 200 mcg and 0.5% in the placebo group.
Postmarketing Experience
- There are no postmarketing adverse experiences reported to date with ASMANEX HFA. However, the postmarketing safety experience with mometasone furoate dry powder inhaler is relevant to ASMANEX HFA since they contain the same active ingredient. The following adverse reactions have been reported during post-approval use of mometasone furoate dry powder inhaler. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Immune System Disorders: Immediate and delayed hypersensitivity reactions including rash, pruritus, angioedema and anaphylactic reaction.
- Respiratory, Thoracic and Mediastinal Disorders: Asthma aggravation, which may include cough, dyspnea, wheezing and bronchospasm.
Drug Interactions
- In clinical trials, concurrent administration of ASMANEX HFA and other drugs, such as short-acting beta2-agonist and intranasal corticosteroids have not resulted in an increased frequency of adverse drug reactions. No formal drug interaction studies have been performed with ASMANEX HFA.
Inhibitors of Cytochrome P450 3A4
- The main route of metabolism of corticosteroids, including mometasone furoate, is via CYP3A4. After oral administration of ketoconazole, a strong inhibitor of CYP3A4, the mean plasma concentration of orally inhaled mometasone furoate increased. Concomitant administration of CYP3A4 inhibitors may inhibit the metabolism of, and increase the systemic exposure to, mometasone furoate. Caution should be exercised when considering the coadministration of ASMANEX HFA with long-term ketoconazole and other known strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin).
Use in Specific Populations
Pregnancy
Teratogenic Effects: Pregnancy Category C
- There are no adequate and well-controlled studies of ASMANEX HFA in pregnant women. Animal reproduction studies of mometasone furoate in mice, rats, and rabbits revealed evidence of teratogenicity. Because animal reproduction studies are not always predictive of human response, ASMANEX HFA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Teratogenic Effects
- When administered to pregnant mice, rats, and rabbits, mometasone furoate increased fetal malformations and decreased fetal growth (measured by lower fetal weights and/or delayed ossification). Dystocia and related complications were also observed when mometasone furoate was administered to rats late in gestation. However, experience with oral corticosteroids suggests that rodents are more prone to teratogenic effects from corticosteroid exposure than humans.
- In a mouse reproduction study, subcutaneous mometasone furoate produced cleft palate at approximately one-third of the maximum recommended daily human dose (MRHD) on a mcg/m2 basis and decreased fetal survival at approximately 1 times the MRHD. No toxicity was observed at approximately one-tenth of the MRHD on a mcg/m2 basis.
- In a rat reproduction study, mometasone furoate produced umbilical hernia at topical dermal doses approximately 6 times the MRHD on a mcg/m2 basis and delays in ossification at approximately 3 times the MRHD on a mcg/m2 basis.
- In another study, rats received subcutaneous doses of mometasone furoate throughout pregnancy or late in gestation. Treated animals had prolonged and difficult labor, fewer live births, lower birth weight, and reduced early pup survival at a dose that was approximately 8 times the MRHD on an area under the curve (AUC) basis. Similar effects were not observed at approximately 4 times MRHD on an AUC basis.
- In rabbits, mometasone furoate caused multiple malformations (e.g., flexed front paws, gallbladder agenesis, umbilical hernia, hydrocephaly) at topical dermal doses approximately 3 times the MRHD on a mcg/m2 basis. In an oral study, mometasone furoate increased resorptions and caused cleft palate and/or head malformations (hydrocephaly and domed head) at a dose less than the MRHD based on AUC. At a dose approximately 2 times the MRHD based on AUC, most litters were aborted or resorbed.
Nonteratogenic Effects
- Hypoadrenalism may occur in infants born to women receiving corticosteroids during pregnancy. Infants born to mothers taking substantial corticosteroid doses during pregnancy should be monitored for signs of hypoadrenalism.
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mometasone (inhalation) in women who are pregnant.
Labor and Delivery
- There are no adequate and well-controlled human studies that have studied the effects of ASMANEX HFA during labor and delivery.
Nursing Mothers
- It is not known whether ASMANEX HFA is excreted in human milk. Because other corticosteroids are excreted in human milk, caution should be exercised when ASMANEX HFA is administered to a nursing woman.
- Since there are no data from well-controlled human studies on the use of ASMANEX HFA on nursing mothers, a decision should be made whether to discontinue nursing or to discontinue ASMANEX HFA, taking into account the importance of ASMANEX HFA to the mother.
Pediatric Use
- The safety and effectiveness of ASMANEX HFA have been established in patients 12 years of age and older in 2 clinical trials of 12 and 26 weeks in duration. In the 2 clinical trials, 32 patients 12 to 17 years of age were treated with ASMANEX HFA. No overall differences in effectiveness were observed between patients in this age group compared to those observed in patients 18 years of age and older. There were no obvious differences in the type or frequency of adverse drug reactions reported in this age group compared to patients 18 years of age and older. The safety and efficacy of ASMANEX HFA have not been established in children less than 12 years of age.
- Controlled clinical studies have shown that inhaled corticosteroids may cause a reduction in growth velocity in pediatric patients. In these studies, the mean reduction in growth velocity was approximately 1 cm per year (range 0.3 to 1.8 per year) and appears to depend upon dose and duration of exposure. This effect was observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with orally inhaled corticosteroids, including the impact on final adult height, are unknown. The potential for "catch-up" growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied.
- The growth of children and adolescents receiving orally inhaled corticosteroids, including ASMANEX HFA, should be monitored routinely (e.g., via stadiometry). If a child or adolescent on any corticosteroid appears to have growth suppression, the possibility that he/she is particularly sensitive to this effect should be considered. The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the risks associated with alternative therapies. To minimize the systemic effects of orally inhaled corticosteroids, including ASMANEX HFA, each patient should be titrated to his/her lowest effective dose.
Geriatic Use
- A total of 38 patients 65 years of age and older (3 of whom were 75 years and older) have been treated with ASMANEX HFA in 2 clinical trials of 12 and 26 weeks in duration. No overall differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Based on available data for ASMANEX HFA, no adjustment of dosage in geriatric patients is warranted.
Gender
- There is no FDA guidance on the use of Mometasone (inhalation) with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Mometasone (inhalation) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mometasone (inhalation) in patients with renal impairment.
Hepatic Impairment
- Concentrations of mometasone furoate appear to increase with severity of hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Mometasone (inhalation) in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Mometasone (inhalation) in patients who are immunocompromised.
Administration and Monitoring
Administration
- ASMANEX HFA should be administered only by the orally inhaled route [see Instructions for Use in the Patient Information leaflet]. After each dose, the patient should be advised to rinse his/her mouth with water without swallowing.
- The cap from the mouthpiece of the actuator should be removed before using ASMANEX HFA.
- ASMANEX HFA should be primed before using for the first time by releasing 4 test sprays into the air, away from the face, shaking well before each spray. In cases where the inhaler has not been used for more than 5 days, prime the inhaler again by releasing 4 test sprays into the air, away from the face, shaking well before each spray.
- The ASMANEX HFA canister should only be used with the ASMANEX HFA actuator. *The ASMANEX HFA actuator should not be used with any other inhalation drug product. Actuators from other products should not be used with the ASMANEX HFA canister.
Monitoring
- Glaucoma, increased intraocular pressure, and cataracts have been reported following the use of long-term administration of inhaled corticosteroids, including mometasone furoate. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
- Patients requiring oral or other systemic corticosteroids should be weaned slowly from oral or other systemic corticosteroid use after transferring to ASMANEX HFA. Lung function (FEV1 or PEF), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral or other systemic corticosteroids. In addition to monitoring asthma signs and symptoms, patients should be observed for signs and symptoms of adrenal insufficiency such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
IV Compatibility
- There is limited information regarding IV Compatibility of Mometasone (inhalation) in the drug label.
Overdosage
- Chronic overdosage may result in signs/symptoms of hypercorticism.Single oral doses up to 8000 mcg of mometasone furoate have been studied on human volunteers with no adverse reactions reported.
Pharmacology
There is limited information regarding Mometasone (inhalation) Pharmacology in the drug label.
Mechanism of Action
- Mometasone furoate is a corticosteroid demonstrating potent anti-inflammatory activity. The precise mechanism of corticosteroid action on asthma is not known. Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of inhibitory effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation and in the asthmatic response. These anti-inflammatory actions of corticosteroids may contribute to their efficacy in asthma.
- Mometasone furoate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor, which is approximately 12 times that of dexamethasone, 7 times that of triamcinolone acetonide, 5 times that of budesonide, and 1.5 times that of fluticasone. The clinical significance of these findings is unknown.
Structure
- Mometasone furoate, the active component of ASMANEX HFA, is a corticosteroid having the chemical name 9,21-dichloro-11(Beta),17-dihydroxy-16 (alpha)-methylpregna-1,4-diene-3,20-dione 17-(2-furoate) with the following chemical structure:
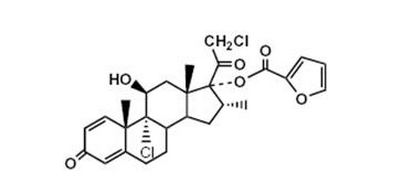
- Mometasone furoate is a white powder with an empirical formula of C27H30Cl2O6, and molecular weight 521.44. It is practically insoluble in water; slightly soluble in methanol, ethanol, and isopropanol; soluble in acetone.
- Each ASMANEX HFA 100 mcg and 200 mcg is a hydrofluoroalkane (HFA-227: 1,1,1,2,3,3,3-heptafluoropropane) propelled pressurized metered dose inhaler containing sufficient amount of drug for 120 actuations. After priming, each actuation of the inhaler delivers 115 or 225 mcg of mometasone furoate in 69.6 mg of suspension from the valve and delivers 100 or 200 mcg of mometasone furoate from the actuator. The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between actuation of the device and inspiration through the delivery system. ASMANEX HFA also contains ethanol as a cosolvent and oleic acid as a surfactant.
- ASMANEX HFA should be primed before using for the first time by releasing 4 test sprays into the air, away from the face, shaking well before each spray. In cases where the inhaler has not been used for more than 5 days, prime the inhaler again by releasing 4 test sprays into the air, away from the face, shaking well before each spray
Pharmacodynamics
HPA Axis Effects
- The effects of inhaled mometasone furoate administered via ASMANEX HFA on adrenal function have not been directly evaluated. However, the effects of inhaled mometasone furoate, administered as part of a mometasone furoate/formoterol fumarate inhalation aerosol combination product, on adrenal function were evaluated in two clinical trials in patients with asthma. As no evidence of a pharmacokinetic drug interaction between mometasone furoate and formoterol was observed when the two drugs were administered in combination, the HPA axis effects from the combination product are applicable to ASMANEX HFA. For the mometasone furoate/formoterol fumarate combination product clinical program, HPA-axis function was assessed by 24-hour plasma cortisol AUC. Although both these trials have open-label design and contain a small number of subjects per treatment arm, results from these trials taken together demonstrated suppression of 24-hour plasma cortisol AUC for the combination mometasone furoate/formoterol fumarate 200 mcg/5 mcg compared to placebo consistent with the known systemic effects of inhaled corticosteroid.
- In a 42-day, open-label, placebo- and active-controlled study, the mean change from baseline plasma cortisol AUC(0-24 hr) was 8%, 22% and 34% lower compared to placebo for the mometasone furoate/formoterol fumarate 100 mcg/5 mcg (n=13), mometasone furoate/formoterol fumarate 200 mcg/5 mcg (n=15) and fluticasone propionate/salmeterol xinafoate 230 mcg/21 mcg (n=16) treatment groups, respectively.
- In a 52-week, open-label safety study, the mean plasma cortisol AUC(0-24 hr) was 2.2%, 29.6%, 16.7%, and 32.2% lower from baseline for the mometasone furoate/formoterol fumarate 100 mcg/5 mcg (n=18), mometasone furoate/formoterol fumarate 200 mcg/5 mcg (n=20), fluticasone propionate/salmeterol xinafoate 125/25 mcg (n=8), and fluticasone propionate/salmeterol xinafoate 250/25 mcg (n=11) treatment groups, respectively.
- The potential effect of mometasone furoate via a dry powder inhaler (DPI) on the HPA axis was also assessed in a 29-day study. A total of 64 adult patients with mild to moderate asthma were randomized to one of 4 treatment groups: mometasone furoate DPI 440 mcg twice daily, mometasone furoate DPI 880 mcg twice daily, oral prednisone 10 mg once daily, or placebo. The 30-minute post-Cosyntropin stimulation serum cortisol concentration on Day 29 was 23.2 mcg/dL for the mometasone furoate DPI 440 mcg twice daily group and 20.8 mcg/dL for the mometasone furoate DPI 880 mcg twice daily group, compared to 14.5 mcg/dL for the oral prednisone 10 mg group and 25 mcg/dL for the placebo group. The difference between mometasone furoate DPI 880 mcg twice daily (twice the maximum recommended dose) and placebo was statistically significant.
Pharmacokinetics
- As no evidence of a pharmacokinetic drug interaction between mometasone furoate and formoterol was observed when the two drugs were administered from a mometasone furoate/formoterol fumarate combination product, the pharmacokinetics information from the combination product is applicable to ASMANEX HFA.
Absorption
- Healthy Subjects: Following oral inhalation of single doses of ASMANEX HFA, mometasone furoate was absorbed in healthy subjects with median Tmax values ranging from 0.50 to 2 hours. Following single-dose administration of higher than recommended dose of ASMANEX HFA (4 inhalations of ASMANEX HFA 200 mcg) in healthy subjects, the arithmetic mean (CV%) Cmax and AUC(0-tf) values for mometasone furoate were 53 (102) pg/mL and 992 (80) pg∙hr/mL, respectively. Studies using oral dosing of labeled and unlabeled drug have demonstrated that the oral systemic bioavailability of mometasone furoate is negligible (<1%).
- Asthma Patients: Following oral inhalation of single and multiple doses of the mometasone furoate/formoterol fumarate combination product, mometasone furoate was absorbed in asthma patients with median Tmax values ranging from 1 to 2 hours. Following single-dose administration of mometasone furoate/formoterol fumarate 400 mcg/10 mcg, the arithmetic mean (CV%) Cmax and AUC(0-12 hr) values for mometasone furoate were 20 (88) pg/mL and 170 (94) pg∙hr/mL, respectively, while the corresponding estimates following twice daily dosing of mometasone furoate/formoterol fumarate 400 mcg/10 mcg at steady-state were 60 (36) pg/mL and 577 (40) pg∙hr/mL.
Distribution
- Based on the study employing a 1000 mcg inhaled dose of tritiated mometasone furoate inhalation powder in humans, no appreciable accumulation of mometasone furoate in the red blood cells was found. Following an intravenous 400 mcg dose of mometasone furoate, the plasma concentrations showed a biphasic decline, with a mean steady-state volume of distribution of 152 liters. The in vitro protein binding for mometasone furoate was reported to be 98% to 99% (in a concentration range of 5 to 500 ng/mL).
Metabolism
- Studies have shown that mometasone furoate is primarily and extensively metabolized in the liver of all species investigated and undergoes extensive metabolism to multiple metabolites. In vitro studies have confirmed the primary role of human liver CYP3A4 in the metabolism of this compound; however, no major metabolites were identified. Human liver CYP3A4 metabolizes mometasone furoate to 6-beta hydroxy mometasone furoate.
Excretion
- Following an intravenous dosing, the terminal half-life was reported to be about 5 hours. Following the inhaled dose of tritiated 1000 mcg mometasone furoate, the radioactivity is excreted mainly in the feces (a mean of 74%), and to a small extent in the urine (a mean of 8%) up to 7 days. No radioactivity was associated with unchanged mometasone furoate in the urine. *Absorbed mometasone furoate is cleared from plasma at a rate of approximately 12.5 mL/min/kg, independent of dose. The effective t½ for mometasone furoate following inhalation with DULERA was 25 hours in healthy subjects and in patients with asthma.
Special Populations
- Hepatic/Renal Impairment: There are no data regarding the specific use of ASMANEX HFA in patients with hepatic or renal impairment.
- A study evaluating the administration of a single inhaled dose of 400 mcg mometasone furoate by a dry powder inhaler to subjects with mild (n=4), moderate (n=4), and severe (n=4) hepatic impairment resulted in only 1 or 2 subjects in each group having detectable peak plasma concentrations of mometasone furoate (ranging from 50-105 pg/mL). The observed peak plasma concentrations appear to increase with severity of hepatic impairment; however, the numbers of detectable levels were few.
- Gender and Race: Specific studies to examine the effects of gender and race on the pharmacokinetics of ASMANEX HFA have not been specifically studied.
- Geriatrics: The pharmacokinetics of ASMANEX HFA have not been specifically studied in the elderly population.
Drug-Drug Interactions
- A single-dose crossover study was conducted to compare the pharmacokinetics of 4 inhalations of the following: mometasone furoate MDI, formoterol MDI, mometasone furoate/formoterol fumarate MDI combination product, and mometasone furoate MDI plus formoterol fumarate MDI administered concurrently. The results of the study indicated that there was no evidence of a pharmacokinetic interaction between mometasone furoate and formoterol.
- Inhibitors of Cytochrome P450 Enzymes: Ketoconazole: In a drug interaction study, an inhaled dose of mometasone furoate 400 mcg delivered by a dry powder inhaler was given to 24 healthy subjects twice daily for 9 days and ketoconazole 200 mg (as well as placebo) were given twice daily concomitantly on Days 4 to 9. Mometasone furoate plasma concentrations were <150 pg/mL on Day 3 prior to coadministration of ketoconazole or placebo. Following concomitant administration of ketoconazole, 4 out of 12 subjects in the ketoconazole treatment group (n=12) had peak plasma concentrations of mometasone furoate >200 pg/mL on Day 9 (211-324 pg/mL). Mometasone furoate plasma levels appeared to increase and plasma cortisol levels appeared to decrease upon concomitant administration of ketoconazole.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a 2-year carcinogenicity study in Sprague Dawley rats, mometasone furoate demonstrated no statistically significant increase in the incidence of tumors at inhalation doses up to 67 mcg/kg (approximately 14 times the MRHD on an AUC basis). In a 19-month carcinogenicity study in Swiss CD-1 mice, mometasone furoate demonstrated no statistically significant increase in the incidence of tumors at inhalation doses up to 160 mcg/kg (approximately 9 times the MRHD on an AUC basis).
- Mometasone furoate increased chromosomal aberrations in an in vitro Chinese hamster ovary cell assay, but did not have this effect in an in vitro Chinese hamster lung cell assay. Mometasone furoate was not mutagenic in the Ames test or mouse lymphoma assay, and was not clastogenic in an in vivo mouse micronucleus assay, a rat bone marrow chromosomal aberration assay, or a mouse male germ-cell chromosomal aberration assay. Mometasone furoate also did not induce unscheduled DNA synthesis in vivo in rat hepatocytes.
- In reproductive studies in rats, impairment of fertility was not produced by subcutaneous doses up to 15 mcg/kg (approximately 8 times the MRHD on an AUC basis).
Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
- In mice, mometasone furoate caused cleft palate at subcutaneous doses of 60 mcg/kg and above (approximately one-third of the maximum recommended human dose MRHD on a mcg/m2 basis). Fetal survival was reduced at 180 mcg/kg (approximately equal to the MRHD on a mcg/m2 basis). No toxicity was observed at 20 mcg/kg (approximately one-tenth of the MRHD on a mcg/m2 basis).
- In rats, mometasone furoate produced umbilical hernia at topical dermal doses of 600 mcg/kg and above (approximately 6 times the MRHD on a mcg/m2 basis). A dose of 300 mcg/kg (approximately 3 times the MRHD on a mcg/m2 basis) produced delays in ossification, but no malformations.
- When rats received subcutaneous doses of mometasone furoate throughout pregnancy or during the later stages of pregnancy, 15 mcg/kg (approximately 8 times the MRHD on an AUC basis) caused prolonged and difficult labor and reduced the number of live births, birth weight, and early pup survival. Similar effects were not observed at 7.5 mcg/kg (approximately 4 times the MRHD on an AUC basis).
- In rabbits, mometasone furoate caused multiple malformations (e.g., flexed front paws, gallbladder agenesis, umbilical hernia, hydrocephaly) at topical dermal doses of 150 mcg/kg and above (approximately 3 times the MRHD on a mcg/m2 basis). In an oral study, mometasone furoate increased resorptions and caused cleft palate and/or head malformations (hydrocephaly and domed head) at 700 mcg/kg (less than the MRHD on an AUC basis). At 2800 mcg/kg (approximately 2 times the MRHD on an AUC basis) most litters were aborted or resorbed. No toxicity was observed at 140 mcg/kg (less than the MRHD on an AUC basis).
Clinical Studies
Asthma
- The safety and efficacy of ASMANEX HFA was demonstrated in two randomized, double-blind, placebo- or active-controlled multi-center clinical trials of 12 and 26 weeks' duration, conducted as part of a mometasone furoate/formoterol fumarate 100/5 mcg or 200/5 mcg combination product development program. A total of 1509 patients 12 years of age and older with persistent asthma (mean baseline FEV1 of 66% to 73% predicted) were evaluated.
Trial 1: Clinical Trial with ASMANEX HFA 100 mcg
- This 26-week, placebo-controlled trial conducted as part of a mometasone furoate/formoterol fumarate combination product asthma program evaluated 781 patients 12 years of age and older. Of these patients, 192 patients received ASMANEX HFA 100 mcg and 196 patients received placebo, each administered as 2 inhalations twice daily by metered dose inhalation aerosols. All other maintenance therapies were discontinued. The study included a 2- to 3-week run-in period with ASMANEX HFA 100 mcg, 2 inhalations twice daily. Patients ranged from 12 to 76 years of age, 41% were male and 59% female, and 72% were Caucasian and 28% non-Caucasian. Patients had persistent asthma and were not well controlled on medium dose of inhaled corticosteroids prior to randomization. Mean FEV1 and mean percent predicted FEV1 were similar among all treatment groups (2.33 L, 73%). Thirteen (7%) patients receiving ASMANEX HFA 100 mcg and 46 (23%) patients receiving placebo discontinued the study early due to treatment failure.
- The change in mean trough FEV1 from baseline to Week 12 compared to placebo was assessed to evaluate the efficacy of ASMANEX HFA 100 mcg. The change from baseline to week 12 in the mean trough FEV1 was greater among patients receiving ASMANEX HFA 100 mcg 2 inhalations twice daily than among those receiving placebo (treatment difference from placebo 0.12 L and 95% confidence interval [0.05, 0.20]).
- Clinically judged deteriorations in asthma or reductions in lung function were also assessed to evaluate the efficacy of ASMANEX HFA 100 mcg. Deteriorations in asthma were defined as any of the following: a 20% decrease in FEV1; a 30% decrease in PEF on two or more consecutive days; emergency treatment, hospitalization, or treatment with systemic corticosteroids or other asthma medications not allowed per protocol. Sixty-five (34%) patients who received ASMANEX HFA 100 mcg reported an event compared to 109 (56%) patients who received placebo.
- Treatment of asthma patients with ASMANEX HFA 100 mcg, two inhalations twice daily also resulted in fewer nocturnal awakenings and improved morning peak flow compared to those who received placebo.
Trial 2: Clinical Trial with ASMANEX HFA 200 mcg
- This 12-week randomized, double-blind, active-controlled trial also conducted as part of a mometasone furoate/formoterol fumarate combination product asthma program evaluated a total of 728 patients 12 years of age and older comparing ASMANEX HFA 200 mcg (n=240 patients), mometasone furoate/formoterol fumarate 200 mcg/5 mcg (n=255 patients), and mometasone furoate/formoterol fumarate 100 mcg/5 mcg (n=233 patients), each administered as 2 inhalations twice daily by metered dose inhalation aerosols. All other maintenance therapies were discontinued. This trial included a 2- to 3-week run-in period with ASMANEX HFA 200 mcg, 2 inhalations twice daily. Patients had persistent asthma and were uncontrolled on high-dose inhaled corticosteroids prior to study entry. Patients ranged from 12 to 84 years of age, 44% were male and 56% female, and 89% were Caucasian and 11% non-Caucasian. Mean FEV1 and mean percent predicted FEV1 values were similar among all treatment groups (2.05 L, 66%). The number of patients who discontinued the trial early due to treatment failure were 11 (5%) in the mometasone furoate/formoterol fumarate 100 mcg/5 mcg group, 8 (3%) in the mometasone furoate/formoterol fumarate 200 mcg/5 mcg group, and 13 (5%) in the ASMANEX HFA 200 mcg group.
- In order to assess the added benefit of a higher dose of mometasone in the 200 mcg/actuation mometasone furoate product compared to the lower dose 100 mcg/actuation product, trough FEV1 at 12 weeks was compared between the combination mometasone furoate/formoterol fumarate 200 mcg/5 mcg and 100 mcg/5 mcg treatment groups as a secondary endpoint. Improvement in trough FEV1 from baseline to week 12 in patients who received mometasone furoate 200 mcg in combination with formoterol fumarate 5 mcg was numerically greater than among patients who received mometasone furoate 100 mcg in combination with formoterol fumarate 5 mcg (treatment difference of 0.05 L and 95% confidence interval [-0.02, 0.10]).
=Other Studies=
- In addition to Trial 1 and Trial 2, the safety and efficacy of mometasone furoate MDI 100 mcg and 200 mcg, in comparison to placebo were demonstrated in three other 12-week, placebo-controlled trials which evaluated the mean change in FEV1 from baseline as a primary endpoint.
How Supplied
- ASMANEX HFA is available in two strengths and supplied in the following package size (Table 4):
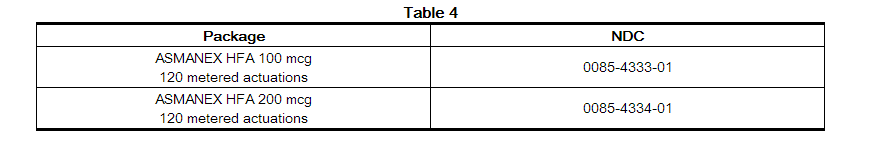
- Each strength is supplied as a pressurized aluminum canister that has a blue plastic actuator integrated with a dose counter and a pink dust cap. Each canister has a net fill weight of 13 grams. Each inhaler is placed into a carton. Each carton contains 1 inhaler.
- Initially the dose counter will display "124" actuations. After the initial priming with 4 actuations, the dose counter will read "120" and the inhaler is now ready for use.
Storage
- The ASMANEX HFA canister should only be used with the ASMANEX HFA actuator. *The ASMANEX HFA actuator should not be used with any other inhalation drug product. Actuators from other products should not be used with the ASMANEX HFA canister.
- The correct amount of medication in each inhalation cannot be ensured after the labeled number of actuations from the canister has been used, even though the inhaler may not feel completely empty and may continue to operate. The inhaler should be discarded when the labeled number of actuations has been used (the dose counter will read "0").
- Store at controlled room temperature 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
- For best results, the canister should be at room temperature before use. Shake well and remove the cap from the mouthpiece of the actuator before using. Keep out of reach of children. Avoid spraying in eyes.
- Contents Under Pressure: Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw container into fire or incinerator.
Images
Drug Images
{{#ask: Page Name::Mometasone (inhalation) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mometasone (inhalation) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Mometasone (inhalation) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[2]
Look-Alike Drug Names
- A® — B®[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Nayak AS, Settipane GA, Pedinoff A, Charous BL, Meltzer EO, Busse WW; et al. (2002). "Effective dose range of mometasone furoate nasal spray in the treatment of acute rhinosinusitis". Ann Allergy Asthma Immunol. 89 (3): 271–8. doi:10.1016/S1081-1206(10)61954-0. PMID 12269647.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Mometasone (inhalation)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}