Methysergide nonclinical toxicology
Jump to navigation
Jump to search
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Animal Pharmacology And Toxicology
Serotonin Antagonism
Considering structure/effect relationships, it has been demonstrated that methylation of the indole nitrogen in the lysergic acid ring of the alkanolamides fundamentally alters their pharmacologic behavior and is associated with inhibition or blockade of a great variety of serotonin-induced effects:
 |
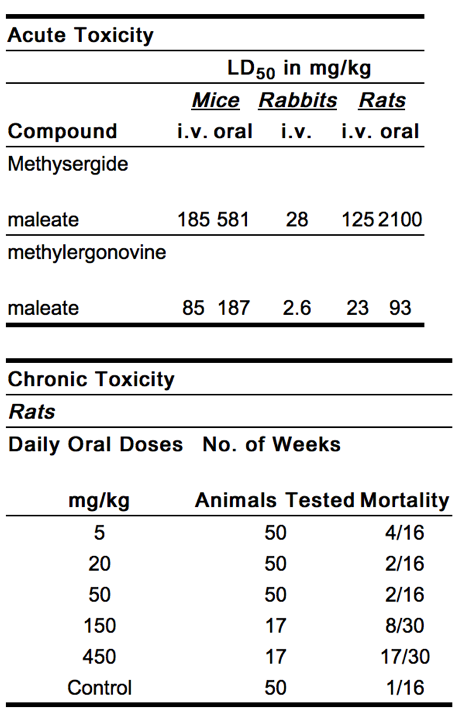 |
Dogs
Oral administration of 1, 2, and 5 mg/kg/day of methysergide maleate failed to produce any major signs of toxicity over a period of 6 months.[1]
References
Adapted from the FDA Package Insert.