Lutetium (177Lu) vipivotide tetraxetan
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lutetium (177Lu) vipivotide tetraxetan is a radioligand therapeutic agent that is FDA approved for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy. Common adverse reactions include fatigue, dry mouth, nausea, anemia, decreased appetite, and constipation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
The recommended PLUVICTO dosage is 7.4 GBq (200 mCi) intravenously every 6 weeks for up to 6 doses, or until disease progression, or unacceptable toxicity.
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Lutetium (177Lu) vipivotide tetraxetan FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
None.
Warnings
Risk From Radiation Exposure
- PLUVICTO contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer.
- Minimize radiation exposure to patients, medical personnel, and household contacts during and after treatment with PLUVICTO consistent with institutional good radiation safety practices, patient treatment procedures, Nuclear Regulatory Commission patient-release guidance, and instructions to the patient for follow-up radiation protection at home.
- Ensure patients increase oral fluid intake and advise patients to void as often as possible to reduce bladder radiation.
- Before the patient is released, the healthcare provider should explain the necessary radioprotection precautions that the patient should follow to minimize radiation exposure to others [see Patient Counseling Information (17)]. Following administration of PLUVICTO, advise patients to limit close contact (less than 3 feet) with household contacts for 2 days or with children and pregnant women for 7 days. Following administration of PLUVICTO, advise patients to refrain from sexual activity for 7 days. Following administration of PLUVICTO, advise patients to sleep in a separate bedroom from household contacts for 3 days, from children for 7 days, or from pregnant women for 15 days.
Myelosuppression
- PLUVICTO can cause severe and life-threatening myelosuppression, including anemia, thrombocytopenia, leukopenia, and neutropenia. In the VISION study, Grade 3 or 4 decreased hemoglobin (15%), decreased platelets (9%), decreased leukocytes (7%), and decreased neutrophils (4.5%) occurred in patients treated with PLUVICTO. Grade ≥ 3 pancytopenia occurred in 1.1% (which includes two fatal events) in patients treated with PLUVICTO. Two deaths (0.4%) due to intracranial hemorrhage and subdural hematoma in association with thrombocytopenia were observed in patients who received PLUVICTO. One death due to sepsis and concurrent neutropenia was observed in patients who received PLUVICTO.
- Perform complete blood counts before and during treatment with PLUVICTO. Withhold, reduce dose, or permanently discontinue PLUVICTO and clinically treat patients based on the severity of myelosuppression.
Renal Toxicity
- PLUVICTO can cause severe renal toxicity. In the VISION study, Grade 3 or 4 acute kidney injury (3%) and increased creatinine (0.9%) occurred in patients treated with PLUVICTO.
- Advise patients to remain well hydrated and to urinate frequently before and after administration of PLUVICTO. Perform kidney function laboratory tests, including serum creatinine and calculated CLcr, before and during treatment with PLUVICTO. Withhold, reduce dose, or permanently discontinue PLUVICTO based on the severity of renal toxicity.
Embryo-Fetal Toxicity
- The safety and efficacy of PLUVICTO have not been established in females. Based on its mechanism of action, PLUVICTO can cause fetal harm. No animal studies using lutetium Lu 177 vipivotide tetraxetan have been conducted to evaluate its effect on female reproduction and embryo-fetal development; however, all radiopharmaceuticals, including PLUVICTO, have the potential to cause fetal harm. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with PLUVICTO and for 14 weeks after the last dose.
Infertility
- PLUVICTO may cause infertility in males. The recommended cumulative dose of 44.4 GBq of PLUVICTO results in a radiation absorbed dose to the testes within the range where PLUVICTO may cause temporary or permanent infertility.
Adverse Reactions
Clinical Trials Experience
- Myelosuppression
- Renal Toxicity
Postmarketing Experience
There is limited information regarding Lutetium (177Lu) vipivotide tetraxetan Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Lutetium (177Lu) vipivotide tetraxetan Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Lutetium (177Lu) vipivotide tetraxetan in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lutetium (177Lu) vipivotide tetraxetan in women who are pregnant.
Labor and Delivery
Risk Summary
The safety and efficacy of PLUVICTO have not been established in females. Based on its mechanism of action, PLUVICTO can cause fetal harm. There are no available data on PLUVICTO use in pregnant females. No animal studies using lutetium Lu 177 vipivotide tetraxetan have been conducted to evaluate its effect on female reproduction and embryo-fetal development; however, all radiopharmaceuticals, including PLUVICTO, have the potential to cause fetal harm.
Nursing Mothers
Risk Summary
The safety and efficacy of PLUVICTO have not been established in females. There are no data on the presence of lutetium Lu 177 vipivotide tetraxetan in human milk or its effects on the breastfed child or on milk production.
Pediatric Use
The safety and effectiveness of PLUVICTO in pediatric patients have not been established.
Geriatic Use
Of the 529 patients who received at least one dose of PLUVICTO plus BSoC in the VISION study, 387 patients (73%) were 65 years or older and 143 patients (27%) were 75 years or older. No overall differences in effectiveness were observed between patients ≥ 75 years of age and younger patients. Serious adverse reactions occurred in 11% of patients ≥ 75 years of age and in 11% of younger patients. Grade ≥ 3 adverse reactions occurred in 40% of patients ≥ 75 years of age and in 31% of younger patients.
Gender
There is no FDA guidance on the use of Lutetium (177Lu) vipivotide tetraxetan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lutetium (177Lu) vipivotide tetraxetan with respect to specific racial populations.
Renal Impairment
Exposure of lutetium Lu 177 vipivotide tetraxetan is expected to increase with the degree of renal impairment [see Clinical Pharmacology (12.3)]. No dose adjustment is recommended for patients with mild (baseline CLcr 60 to 89 mL/min by Cockcroft-Gault) to moderate (CLcr 30 to 59 mL/min) renal impairment; however, patients with mild or moderate renal impairment may be at greater risk of toxicity. Frequently monitor renal function and adverse reactions in patients with mild to moderate renal impairment [see Dosage and Administration (2.4)]. The pharmacokinetics and safety of PLUVICTO have not been studied in patients with severe (CLcr 15 to 29 mL/min) renal impairment or end-stage renal disease.
Hepatic Impairment
There is no FDA guidance on the use of Lutetium (177Lu) vipivotide tetraxetan in patients with hepatic impairment.
Females of Reproductive Potential and Males
Contraception
Males
Based on its mechanism of action, advise male patients with female partners of reproductive potential to use effective contraception during treatment with PLUVICTO and for 14 weeks after the last dose [see Clinical Pharmacology (12.1), Nonclinical Toxicology (13.1)].
Infertility
The recommended cumulative dose of 44.4 GBq of PLUVICTO results in a radiation absorbed dose to the testes within the range where PLUVICTO may cause temporary or permanent infertility.
Immunocompromised Patients
There is no FDA guidance one the use of Lutetium (177Lu) vipivotide tetraxetan in patients who are immunocompromised.
Administration and Monitoring
Administration
Preparation Instructions
Use aseptic technique and radiation shielding when handling or administering PLUVICTO, using tongs as needed to minimize radiation exposure. Inspect the vial visually under a shielded screen for particulate matter and discoloration prior to administration. Discard the vial if particulates or discoloration are present. Do not inject the PLUVICTO solution directly into any other intravenous solution. Confirm the amount of radioactivity delivered to the patient with an appropriately calibrated dose calibrator prior to and after PLUVICTO administration. Dispose of any unused medicinal product or waste material in accordance with local and federal laws. Administration Instructions
The recommended dosage of PLUVICTO may be administered intravenously as an injection using a disposable syringe fitted with a syringe shield (with or without a syringe pump), as an infusion using the gravity method (with or without an infusion pump), or as an infusion using the vial (with a peristaltic infusion pump).
A reduced dose of PLUVICTO should be administered using the syringe method (with or without a syringe pump) or the vial method (with a peristaltic infusion pump). Using the gravity method to administer a reduced dose of PLUVICTO is not recommended since it may result in delivery of the incorrect volume of PLUVICTO, if the dose is not adjusted prior to administration.
Prior to administration, flush the intravenous catheter used exclusively for PLUVICTO administration with ≥ 10 mL of 0.9% sterile sodium chloride solution to ensure patency and to minimize the risk of extravasation. Manage cases of extravasation as per institutional guidelines.
Instructions for the Syringe Method (With or Without a Syringe Pump)
After disinfecting the vial stopper, withdraw an appropriate volume of PLUVICTO solution to deliver the desired radioactivity by using a disposable syringe fitted with a syringe shield and a disposable sterile needle. Administer PLUVICTO to the patient by slow intravenous push within approximately 1 to 10 minutes (either with a syringe pump or manually without a syringe pump) via an intravenous catheter that is pre-filled with 0.9% sterile sodium chloride solution and that is used exclusively for PLUVICTO administration to the patient. Once the desired PLUVICTO radioactivity has been administered, perform an intravenous flush of ≥ 10 mL of 0.9% sterile sodium chloride solution through the intravenous catheter to the patient. Instructions for the Gravity Method (With or Without an Infusion Pump)
Insert a 2.5 cm, 20 gauge needle (short needle) into the PLUVICTO vial and connect via a catheter to 500 mL 0.9% sterile sodium chloride solution (used to transport the PLUVICTO solution during the infusion). Ensure that the short needle does not touch the PLUVICTO solution in the vial and do not connect the short needle directly to the patient. Do not allow the sodium chloride solution to flow into the PLUVICTO vial prior to the initiation of the PLUVICTO infusion and do not inject the PLUVICTO solution directly into the sodium chloride solution. Insert a second needle that is 9 cm, 18 gauge (long needle) into the PLUVICTO vial, ensuring that the long needle touches and is secured to the bottom of the PLUVICTO vial during the entire infusion. Connect the long needle to the patient by an intravenous catheter that is pre-filled with 0.9% sterile sodium chloride solution and that is used exclusively for the PLUVICTO infusion into the patient. Use a clamp or an infusion pump to regulate the flow of the sodium chloride solution via the short needle into the PLUVICTO vial (the sodium chloride solution entering the vial through the short needle will carry the PLUVICTO solution from the vial to the patient via the intravenous catheter connected to the long needle within approximately 30 minutes). During the infusion, ensure that the level of solution in the PLUVICTO vial remains constant. Disconnect the vial from the long needle line and clamp the saline line once the level of radioactivity is stable for at least five minutes. Follow the infusion with an intravenous flush of ≥ 10 mL of 0.9% sterile sodium chloride solution through the intravenous catheter to the patient. Instructions for the Vial Method (With a Peristaltic Infusion Pump)
Insert a 2.5 cm, 20 gauge needle (short venting needle) into the PLUVICTO vial. Ensure that the short needle does not touch the PLUVICTO solution in the vial and do not connect the short needle directly to the patient or to the peristaltic infusion pump. Insert a second needle that is 9 cm, 18 gauge (long needle) into the PLUVICTO vial, ensuring that the long needle touches and is secured to the bottom of the PLUVICTO vial during the entire infusion. Connect the long needle and a 0.9% sterile sodium chloride solution to a 3-way stopcock valve via appropriate tubing. Connect the output of the 3-way stopcock valve to tubing installed on the input side of the peristaltic infusion pump following the pump manufacturer’s instructions. Pre-fill the line by opening the 3-way stopcock valve and pumping the PLUVICTO solution through the tubing until it reaches the exit of the valve. Pre-fill the intravenous catheter which will be connected to the patient by opening the 3-way stopcock valve to the 0.9% sterile sodium chloride solution and pumping the 0.9% sterile sodium chloride solution until it exits the end of the catheter tubing. Connect the pre-filled intravenous catheter to the patient and set the 3-way stopcock valve such that the PLUVICTO solution is in line with the peristaltic infusion pump. Infuse an appropriate volume of PLUVICTO solution at approximately 25 mL/h to deliver the desired radioactivity. When the desired PLUVICTO radioactivity has been delivered, stop the peristaltic infusion pump and then change the position of the 3-way stopcock valve so that the peristaltic infusion pump is in line with the 0.9% sterile sodium chloride solution. Restart the peristaltic infusion pump and infuse an intravenous flush of ≥ 10 mL of 0.9% sterile sodium chloride solution through the intravenous catheter to the patient.
Monitoring
There is limited information regarding Lutetium (177Lu) vipivotide tetraxetan Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Lutetium (177Lu) vipivotide tetraxetan and IV administrations.
Overdosage
In the event of administration of a radiation overdosage with PLUVICTO, reduce the radiation absorbed dose to the patient by increasing the elimination of the radionuclide from the body by frequent micturition or by forced diuresis and frequent bladder voiding. Estimate the effective radiation dose that was applied and treat with additional supportive care measures as clinically indicated.
Pharmacology
Mechanism of Action
Lutetium Lu 177 vipivotide tetraxetan is a radioligand therapeutic agent. The active moiety of lutetium Lu 177 vipivotide tetraxetan is the radionuclide lutetium-177 which is linked to a moiety that binds to PSMA, a transmembrane protein that is expressed in prostate cancer, including mCRPC. Upon binding of lutetium Lu 177 vipivotide tetraxetan to PSMA-expressing cells, the beta-minus emission from lutetium-177 delivers radiation to PSMA-expressing cells, as well as to surrounding cells, and induces DNA damage which can lead to c
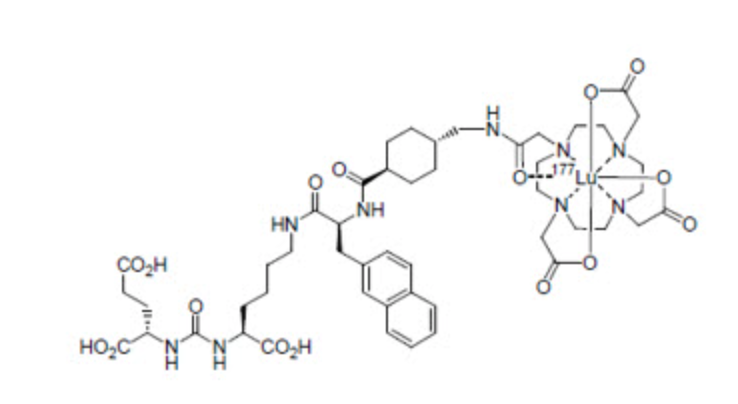
Structure
PLUVICTO (lutetium Lu 177 vipivotide tetraxetan) is a radioligand therapeutic agent. Lutetium Lu 177 vipivotide tetraxetan is a PSMA-binding ligand bound to a DOTA chelator radiolabeled with lutetium-177.
The molecular mass is 1216.06 g/mol and the molecular formula is C49H68177LuN9O16.
PLUVICTO (lutetium Lu 177 vipivotide tetraxetan) 1,000 MBq/mL (27 mCi/mL) Injection is supplied as a sterile, clear, colorless to slightly yellow solution for intravenous use. Each single-dose vial contains acetic acid (0.30 mg/mL), sodium acetate (0.41 mg/mL), gentisic acid (0.39 mg/mL), sodium ascorbate (50.0 mg/mL), pentetic acid (0.10 mg/mL), and water for injection (q.s. to 1 mL). The pH range of the solution is 4.5 to 7.0.
Pharmacodynamics
Lutetium Lu 177 vipivotide tetraxetan exposure-efficacy relationships and the time course of pharmacodynamic response have not been fully characterized.
Cardiac Electrophysiology
At the recommended dosage, PLUVICTO does not cause large mean increases (> 20 ms) in the QTc interval.
Pharmacokinetics
Pharmacokinetics of lutetium Lu 177 vipivotide tetraxetan are expressed as geometric mean (geometric mean coefficient of variation) unless otherwise specified.
The blood lutetium Lu 177 vipivotide tetraxetan area under the curve (AUC) is 52.3 ng.h/mL (31.4%) and the maximum blood concentration is 6.58 ng/mL (43.5%) at the recommended dosage.
Distribution
Lutetium Lu 177 vipivotide tetraxetan volume of distribution is 123 L (78.1%).
Within 2.5 hours of administration, lutetium Lu 177 vipivotide tetraxetan distributes to gastrointestinal tract, liver, lungs, kidneys, heart wall, bone marrow, and salivary glands.
Vipivotide tetraxetan and non-radioactive lutetium vipivotide tetraxetan are 60% to 70% bound to human plasma proteins.
Elimination
The lutetium Lu 177 vipivotide tetraxetan terminal elimination half-life is 41.6 hours (68.8%) and the clearance (CL) is 2.04 L/h (31.5%).
Excretion
Lutetium Lu 177 vipivotide tetraxetan is primarily eliminated renally.
Specific Populations
Exposure (AUC) of lutetium Lu 177 vipivotide tetraxetan increased with decreasing creatinine clearance (CLcr). The effect of baseline CLcr < 54 mL/min on lutetium Lu 177 vipivotide tetraxetan pharmacokinetics has not been studied.
Drug Interaction Studies
In Vitro Studies
CYP450 enzymes: Vipivotide tetraxetan is not a substrate of cytochrome P450 (CYP450) enzymes. Vipivotide tetraxetan did not induce CYP1A2, 2B6 or 3A4; and did not inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 or 3A in vitro.
Transporters: Vipivotide tetraxetan is not a substrate of BCRP, P-gp, MATE1, MATE2-K, OAT1, OAT3 or OCT2. Vipivotide tetraxetan did not inhibit BCRP, P-gp, MATE1, MATE2-K, OAT1, OAT3, OATP1B1, OATP1B3, OCT1 or OCT2 in vitro.
Nonclinical Toxicology
Pharmacokinetics of lutetium Lu 177 vipivotide tetraxetan are expressed as geometric mean (geometric mean coefficient of variation) unless otherwise specified.
The blood lutetium Lu 177 vipivotide tetraxetan area under the curve (AUC) is 52.3 ng.h/mL (31.4%) and the maximum blood concentration is 6.58 ng/mL (43.5%) at the recommended dosage.
Distribution
Lutetium Lu 177 vipivotide tetraxetan volume of distribution is 123 L (78.1%).
Within 2.5 hours of administration, lutetium Lu 177 vipivotide tetraxetan distributes to gastrointestinal tract, liver, lungs, kidneys, heart wall, bone marrow, and salivary glands.
Vipivotide tetraxetan and non-radioactive lutetium vipivotide tetraxetan are 60% to 70% bound to human plasma proteins.
Elimination
The lutetium Lu 177 vipivotide tetraxetan terminal elimination half-life is 41.6 hours (68.8%) and the clearance (CL) is 2.04 L/h (31.5%).
Excretion
Lutetium Lu 177 vipivotide tetraxetan is primarily eliminated renally.
Specific Populations
Exposure (AUC) of lutetium Lu 177 vipivotide tetraxetan increased with decreasing creatinine clearance (CLcr). The effect of baseline CLcr < 54 mL/min on lutetium Lu 177 vipivotide tetraxetan pharmacokinetics has not been studied.
Drug Interaction Studies
In Vitro Studies
CYP450 enzymes: Vipivotide tetraxetan is not a substrate of cytochrome P450 (CYP450) enzymes. Vipivotide tetraxetan did not induce CYP1A2, 2B6 or 3A4; and did not inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 or 3A in vitro.
Transporters: Vipivotide tetraxetan is not a substrate of BCRP, P-gp, MATE1, MATE2-K, OAT1, OAT3 or OCT2. Vipivotide tetraxetan did not inhibit BCRP, P-gp, MATE1, MATE2-K, OAT1, OAT3, OATP1B1, OATP1B3, OCT1 or OCT2 in vitro.
Clinical Studies
The efficacy of PLUVICTO was evaluated in VISION (NCT03511664), a randomized (2:1), multicenter, open-label trial that evaluated PLUVICTO plus BSoC (N = 551) or BSoC alone (N = 280) in men with progressive, PSMA-positive mCRPC. Randomization was stratified by baseline lactase dehydrogenase (LDH), presence of liver metastases, ECOG PS score and inclusion of an AR pathway inhibitor as part of BSoC at the time of randomization. All patients received a GnRH analog or had prior bilateral orchiectomy. Patients were required to have received at least one AR pathway inhibitor, and 1 or 2 prior taxane-based chemotherapy regimens. Eligible patients were required to have PSMA-positive mCRPC defined as having at least one tumor lesion with gallium Ga 68 gozetotide uptake greater than normal liver. Patients were excluded if any lesions exceeding size criteria in short axis [organs ≥ 1 cm, lymph nodes ≥ 2.5 cm, bones (soft tissue component) ≥ 1 cm] had uptake less than or equal to uptake in normal liver.
Patients received PLUVICTO 7.4 GBq (200 mCi) every 6 weeks for up to a total of 6 doses plus BSoC or BSoC alone. BSoC administered at the investigator’s discretion included ketoconazole; radiation therapy to localized prostate cancer targets; bone-targeted agents; androgen-reducing agents; AR pathway inhibitors. Patients continued treatment for up to 4-6 doses, or until disease progression or unacceptable toxicity. Patients with stable disease or partial response after 4 doses of PLUVICTO plus BSoC received up to 2 additional doses per investigator’s discretion.
The following patient demographics and baseline disease characteristics were balanced between the arms. The median age was 71 years (range, 40 to 94 years); 87% White; 7% Black or African American; 2.4% Asian; 92% had ECOG PS0-1; 8% had ECOG PS2. All patients had received at least one prior taxane-based chemotherapy regimen and 41% of patients received two. One prior AR pathway inhibitor had been administered to 51% of patients, 41% of patients received 2, and 8% of patients received 3 or more. During the treatment period, 53% of patients in the PLUVICTO plus BSoC arm and 68% of patients in the BSoC alone arm received at least one AR pathway inhibitor.
The major efficacy outcome measures were overall survival (OS) and radiographic progression-free survival (rPFS) by blinded independent central review (BICR) per Prostate Cancer Working Group 3 (PCWG3) criteria. An additional efficacy outcome measure included was overall response rate (ORR) by BICR per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
VISION demonstrated a statistically significant improvement in both major efficacy outcome measures of OS and rPFS by BICR with PLUVICTO plus BSoC compared to treatment with BSoC alone. Interpretation of the magnitude of the rPFS effect was limited due to a high degree of censoring from early drop out in the control arm.
How Supplied
PLUVICTO Injection containing 1,000 MBq/mL (27 mCi/mL) of lutetium Lu 177 vipivotide tetraxetan is a sterile, preservative-free and clear, colorless to slightly yellow solution for intravenous use supplied in a colorless type I glass 30 mL single-dose vial containing 7.4 GBq (200 mCi) ± 10% of lutetium Lu 177 vipivotide tetraxetan at the date and time of administration (NDC# 69488-010-61). The solution volume in the vial can range from 7.5 mL to 12.5 mL in order to provide a total of 7.4 GBq (200 mCi) of radioactivity at the date and time of administration.
The product vial is in a lead shielded container (NDC# 69488-010-61) placed in a plastic sealed container. The product is shipped in a type A package (NDC# 69488-010-61).
The shelf life is 120 hours (5 days) from the date and time of calibration.
Storage
Store below 30°C (86°F). Do not freeze. Store in the original package to protect from ionizing radiation (lead shielding).
Store PLUVICTO in accordance with local and federal laws on radioactive materials.
Do not use PLUVICTO after the expiration date and time which are stated on the label.
Dispose of any unused medicinal product or waste material in accordance with local and federal laws.
Lutetium-177 may be prepared using two different sources of stable isotopes (either lutetium-176 or ytterbium-176) that require different waste management. Lutetium-177 is prepared using ytterbium-176 (“non-carrier added”) unless otherwise communicated on the product batch release certificate.
Images
Drug Images
{{#ask: Page Name::Lutetium (177Lu) vipivotide tetraxetan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PLUVICTO®
1,000 MBq/mL (27 mCi/mL)
lutetium Lu 177 vipivotide tetraxetan injection
Intravenous use Single-dose vial
Sterile
NDC# 69488-010-61
Rx Only
Advanced Accelerator Applications USA, Inc. Millburn, NJ 07041

{{#ask: Label Page::Lutetium (177Lu) vipivotide tetraxetan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Risk From Radiation Exposure
- Ensure patients increase oral fluid intake and advise patients to void as often as possible to reduce bladder radiation.
- Explain the necessary radioprotection precautions that the patient should follow to minimize radiation exposure to others before the patient is released. Following administration of PLUVICTO, advise patients to limit close contact (less than 3 feet) with household contacts for 2 days or with children and pregnant women for 7 days. Following administration of PLUVICTO, advise patients to refrain from sexual activity for 7 days. Following administration of PLUVICTO, advise patients to sleep in a separate bedroom from household contacts for 3 days, from children for 7 days, or from pregnant women for 15 days.
Myelosuppression
- Advise patients to contact their healthcare provider for any signs or symptoms of myelosuppression, such as tiredness, weakness, pale skin, shortness of breath, bleeding or bruising more easily than normal or difficulty to stop bleeding, or frequent infections with signs, such as fever, chills, sore throat or mouth ulcers.
Renal Toxicity
- Advise patients to remain well hydrated and to urinate frequently before and after administration of PLUVICTO. Advise patients to contact their healthcare provider for any signs or symptoms of renal toxicity, such as passing urine less often than usual or passing much smaller amounts of urine than usual.
Embryo-Fetal Toxicity
- Advise patients that PLUVICTO can cause fetal harm.
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with PLUVICTO and for 14 weeks after the last dose.
Infertility
- Advise males of reproductive potential that PLUVICTO may cause temporary or permanent infertility.
Precautions with Alcohol
Alcohol-Lutetium (177Lu) vipivotide tetraxetan interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
PLUVICTO
Look-Alike Drug Names
There is limited information regarding Lutetium (177Lu) vipivotide tetraxetan Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Lutetium (177Lu) vipivotide tetraxetan
|Pill Name=Pluvicto structure.png
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Page Name=Lutetium (177Lu) vipivotide tetraxetan
|Pill Name=Pluvicto label.png
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}