Luliconazole
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Luliconazole is a azole antifungal that is FDA approved for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis. Common adverse reactions include application site reactions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- LUZU (luliconazole) Cream, 1% is indicated for the topical treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organisms Trichophyton rubrum and Epidermophyton floccosum, in patients 18 years of age and older.
Dosage
- For topical use only. LUZU Cream, 1% is not for ophthalmic, oral, or intravaginal use.
- When treating interdigital tinea pedis, a thin layer of LUZU Cream, 1% should be applied to the affected area and approximately 1 inch of the immediate surrounding area(s) once daily for two (2) weeks.
- When treating tinea cruris or tinea corporis, LUZU Cream, 1% should be applied to the affected area and approximately 1 inch of the immediate surrounding area(s) once daily for one (1) week.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Luliconazole in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Luliconazole in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- safety and efficacy not established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Luliconazole in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Luliconazole in pediatric patients.
Contraindications
- None
Warnings
There is limited information regarding Luliconazole Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in practice.
- In three Phase 3 clinical trials, 616 subjects were exposed to LUZU Cream, 1%: 305 with interdigital tinea pedis and 311 subjects with tinea cruris. Subjects with interdigital tinea pedis or tinea cruris applied LUZU Cream, 1% or vehicle cream once daily for 14 days or 7 days, respectively, to affected and adjacent areas. During clinical trials with LUZU Cream, 1%, the most common adverse reactions were application site reactions which occurred in less than 1% of subjects in both the LUZU and vehicle arms. Most adverse reactions were mild in severity.
Postmarketing Experience
- The following adverse reactions have been identified during postmarketing use of luliconazole cream, 1%: contact dermatitis and cellulitis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Drug Interactions
- The potential of luliconazole to inhibit cytochrome P-450 (CYP) enzymes 1A2, 2C9, 2C19, 2D6, and 3A4 was evaluated in vitro. Based on in vitro assessment, luliconazole at therapeutic doses, particularly when applied to patients with moderate to severe tinea cruris, may inhibit the activity of CYP2C19 and CYP3A4. However, no in vivo drug interaction trials have been conducted to evaluate the effect of luliconazole on other drugs that are substrates of CYP2C19 and CYP3A4.
- Luliconazole is not expected to inhibit CYPs 1A2, 2C9 and 2D6 based on in vitro assessment. The induction potential of luliconazole on CYP enzymes has not been evaluated.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of LUZU Cream, 1% in pregnant women. LUZU Cream, 1% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- The animal multiples of human exposure calculations were based on daily dose body surface area (BSA) comparisons (mg/m2) for the reproductive toxicology studies described in this section and in Section 13.1. The Maximum Recommended Human Dose (MRHD) was set at 8 g 1% cream per day (1.33 mg/kg/day for a 60 kg individual which is equivalent to 49.2 mg/m2/day).
- Systemic embryofetal development studies were conducted in rats and rabbits. Subcutaneous doses of 1, 5 and 25 mg/kg/day luliconazole were administered during the period of organogenesis (gestational days 7-17) to pregnant female rats. No treatment related effects on maternal toxicity or malformations were noted at 25 mg/kg/day (3 times the MRHD based on BSA comparisons). Increased incidences of skeletal variation (14th rib) were noted at 25 mg/kg/day. No treatment related effects on skeletal variation were noted at 5 mg/kg/day (0.6 times the MRHD based on BSA comparisons).
- Subcutaneous doses of 4, 20 and 100 mg/kg/day luliconazole were administered during the period of organogenesis (gestational days 6-18) to pregnant female rabbits. No treatment related effects on maternal toxicity, embryofetal toxicity or malformations were noted at 100 mg/kg/day (24 times the MRHD based on BSA comparisons).
- In a pre- and post-natal development study in rats, subcutaneous doses of 1, 5 and 25 mg/kg/day luliconazole were administered from the beginning of organogenesis (gestation day 7) through the end of lactation (lactation day 20). In the presence of maternal toxicity, embryofetal toxicity (increased prenatal pup mortality, reduced live litter sizes and increased postnatal pup mortality) was noted at 25 mg/kg/day. No embryofetal toxicity was noted at 5 mg/kg/day (0.6 times the MRHD based on BSA comparisons). No treatment effects on postnatal development were noted at 25 mg/kg/day (3 times the MRHD based on BSA comparisons).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Luliconazole in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Luliconazole during labor and delivery.
Nursing Mothers
- It is not known whether luliconazole is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when LUZU Cream, 1% is administered to women who are breastfeeding.
Pediatric Use
- The safety and effectiveness of LUZU Cream, 1% in pediatric patients have not been established. The number of pediatric subjects ≥12 years of age were too small to adequately assess safety and efficacy.
Geriatic Use
- Of the total number of subjects in clinical studies of LUZU Cream, 1%, 8 percent were 65 and over, while 1.4 percent were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Luliconazole with respect to specific gender populations.
Race
There is no FDA guidance on the use of Luliconazole with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Luliconazole in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Luliconazole in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Luliconazole in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Luliconazole in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Luliconazole in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Luliconazole in the drug label.
Overdosage
There is limited information regarding Overdose of Luliconazole in the drug label.
Pharmacology
| |
Luliconazole
| |
| Systematic (IUPAC) name | |
| (2E)-[(4R)-4-(2,4-Dichlorophenyl)-1,3-dithiolan-2-ylidene](1H-imidazol-1-yl)acetonitrile | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 354.28 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Template:Unicode Prescription only |
| Routes | ? |
Mechanism of Action
- Luliconazole is an antifungal that belongs to the azole class. Although the exact mechanism of action against dermatophytes is unknown, luliconazole appears to inhibit ergosterol synthesis by inhibiting the enzyme lanosterol demethylase. Inhibition of this enzyme’s activity by azoles results in decreased amounts of ergosterol, a constituent of fungal cell membranes, and a corresponding accumulation of lanosterol.
Structure
- LUZU (luliconazole) Cream, 1% contains 1% luliconazole, an azole antifungal agent, in a white cream for topical application.
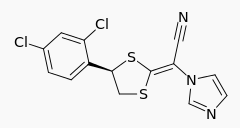
- Luliconazole is (2E)-2-[(4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-ylacetonitrile. Its structural formula is:
- The molecular formula is C14H9Cl2N3S2 with a molecular weight of 354.28. Luliconazole is the R-enantiomer and contains one chiral center. The double bond adjacent to the dithiolane group is in the E configuration.
- LUZU Cream, 1% contains 10 mg of luliconazole per gram of cream in a vehicle consisting of benzyl alcohol, butylated hydroxytoluene, cetostearyl alcohol, isopropyl myristate, medium-chain triglycerides, methylparaben, polysorbate 60, propylene glycol, purified water, and sorbitan monostearate.
Pharmacodynamics
At therapeutic doses, LUZU Cream, 1% is not expected to prolong QTc to any clinically relevant extent.
Pharmacokinetics
- Luliconazole is the R enantiomer of a chiral molecule. The potential for inter-conversion between R and S enantiomers in humans has not been assessed. Information on the pharmacokinetics of luliconazole presented below refers to both R enantiomer and S enantiomer, if any, combined.
- Luliconazole is >99% protein bound in plasma.
- In a pharmacokinetic trial, 12 subjects with moderate to severe tinea pedis and 8 subjects with moderate to severe tinea cruris applied a mean daily amount of approximately 3.5 grams of LUZU Cream, 1% to the affected and surrounding areas once daily for 15 days. Plasma concentrations of luliconazole on Day 15 were measurable in all subjects and fluctuated little during the 24 hour interval. In subjects with tinea pedis, the mean ± SD of the maximum concentration (Cmax) was 0.40 ± 0.76 ng/mL after the first dose and 0.93 ± 1.23 ng/mL after the final dose. The mean time to reach Cmax (Tmax) was 16.9 ± 9.39 hours after the first dose and 5.8 ± 7.61 hours after the final dose. Exposure to luliconazole, as expressed by area under the concentration time curve (AUC0–24 was 6.88 ± 14.50 ng*hr/mL after the first dose and 18.74 ± 27.05 ng*hr/mL after the final dose. In subjects with tinea cruris, the mean ± SD Cmax was 4.91 ± 2.51 ng/mL after the first dose and 7.36 ± 2.66 ng/mL after the final dose. The mean Tmax was 21.0 ± 5.55 hours after the first dose and 6.5 ± 8.25 hours after the final dose. Exposure to luliconazole, as expressed by AUC0–24 was 85.1 ± 43.69 ng*hr/mL after the first dose and 121.74 ± 53.36 ng*hr/mL after the final dose.
Microbiology
Mechanism of Resistance
- To date, a mechanism of resistance to luliconazole has not been described.
- LUZU Cream, 1% has been shown to be active against most isolates of the following fungi, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies to evaluate the carcinogenic potential of LUZU Cream, 1% have not been conducted.
- Luliconazole revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and Chinese hamster lung cell chromosomal aberration assay) and one in vivo genotoxicity test (mouse bone marrow micronucleus test).
- In a fertility study in rats, subcutaneous doses of 1, 5 and 25 mg/kg/day luliconazole were administered prior to and during mating and through early pregnancy. Treatment related effects on reproductive function were noted in females (decreased live embryos and decreased corpus luteum) at 5 and 25 mg/kg/day and males (decreased sperm counts) at 25 mg/kg/day. No treatment related effects on fertility or reproductive function were noted at 1 mg/kg/day (0.1× MRHD based on BSA comparisons).
Clinical Studies
Interdigital Tinea Pedis
- The safety and efficacy of LUZU (luliconazole) Cream, 1% was evaluated in two randomized, double-blind, vehicle-controlled, multi-center clinical trials in 423 subjects with a clinical and culture-confirmed diagnosis of interdigital tinea pedis. Subjects were randomized to receive LUZU Cream, 1% or vehicle. Subjects applied either LUZU Cream, 1% or vehicle cream to the entire area of the forefeet including all interdigital web spaces and approximately 2.5 cm (1 in) of the surrounding area of the foot once daily for 14 days.
- The mean age of the study population was 41 years; 82% were male; 53% were White and 40% were Black or African American. Signs and symptoms of tinea pedis (erythema, scaling, and pruritus), KOH exam and dermatophyte culture were assessed at baseline, end-of-treatment (Day 14), 2 and 4 weeks post-treatment.
- Overall treatment success was defined as complete clearance (clinical cure and mycological cure) at 4 weeks post-treatment. LUZU Cream, 1% demonstrated completed clearance in subjects with interdigital tinea pedis. Treatment outcomes at 4 weeks post-treatment are summarized in Table 1.
Tinea Cruris
- The safety and efficacy of LUZU (luliconazole) Cream, 1% was evaluated in a randomized, double-blind, vehicle-controlled, multi-center clinical trial in 256 subjects with a clinical and culture confirmed diagnosis of tinea cruris. Subjects were randomized to receive LUZU Cream, 1% or vehicle. Subjects applied either LUZU Cream, 1% or vehicle cream to the affected area and approximately 2.5 cm (1 in) of the surrounding area once daily for 7 days.
- The mean age of the study population was 40 years; 83% were male; 58% were White and 34% were Black or African American. Signs and symptoms of tinea cruris (erythema, scaling, and pruritus), positive KOH exam and dermatophyte culture were assessed at baseline, end-of-treatment (Day 7), 2 and 3 weeks post-treatment.
- Overall treatment success was defined as complete clearance (clinical cure and mycological cure) at 3 weeks post-treatment. LUZU Cream, 1% demonstrated completed clearance in subjects with tinea cruris. Treatment outcomes at 3 weeks post treatment are summarized in Table 2.
How Supplied
- LUZU (luliconazole) Cream, 1% is a white cream supplied in tubes as follows:
- 60 g (NDC 99207-850-60)
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F)
Images
Drug Images
{{#ask: Page Name::Luliconazole |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
Ingredients and Appearance
{{#ask: Label Page::Luliconazole |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform patients that LUZU Cream, 1% is for topical use only. LUZU Cream, 1% is not intended for intravaginal or ophthalmic use.
PATIENT INFORMATION
Precautions with Alcohol
- Alcohol-Luliconazole interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- LUZU®[1]
Look-Alike Drug Names
There is limited information regarding look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.





