Lamivudine adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Adverse Reactions
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Lactic acidosis and severe hepatomegaly with steatosis [see Boxed Warning, Warnings and Precautions (5.1)].
- Severe acute exacerbations of hepatitis B [see Boxed Warning, Warnings and Precautions (5.2)].
- Hepatic decompensation in patients co-infected with HIV-1 and hepatitis C [see Warnings and Precautions (5.4)].
- Pancreatitis [see Warnings and Precautions (5.5)].
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults - Clinical Trials in HIV-1: The safety profile of EPIVIR in adults is primarily based on 3,568 HIV-1-infected subjects in 7 clinical trials.
The most common adverse reactions are headache, nausea, malaise, fatigue, nasal signs and symptoms, diarrhea and cough.
Selected clinical adverse reactions in ≥5% of subjects during therapy with EPIVIR 150 mg twice daily plus RETROVIR® 200 mg 3 times daily for up to 24 weeks are listed in Table 3.
 |
Pancreatitis: Pancreatitis was observed in 9 out of 2,613 adult subjects (0.3%) who received EPIVIR in controlled clinical trials EPV20001, NUCA3001, NUCB3001, NUCA3002, NUCB3002, and NUCB3007 [see Warnings and Precautions (5.5)].
EPIVIR 300 mg Once Daily: The types and frequencies of clinical adverse reactions reported in subjects receiving EPIVIR 300 mg once daily or EPIVIR 150 mg twice daily (in 3-drug combination regimens in EPV20001 and EPV40001) for 48 weeks were similar.
Selected laboratory abnormalities observed during therapy are summarized in Table 4.
 |
The frequencies of selected laboratory abnormalities reported subjects receiving EPIVIR 300 mg once daily or EPIVIR 150 mg twice daily (in 3-drug combination regimens in EPV20001 and EPV40001) were similar.
Pediatric Subjects – Clinical Trials in HIV-1: EPIVIR Oral Solution has been studied in 638 pediatric subjects aged 3 months to 18 years in 3 clinical trials.
Selected clinical adverse reactions and physical findings with a ≥5% frequency during therapy with EPIVIR 4 mg/kg twice daily plus RETROVIR 160 mg/m2 3 times daily in therapy-naive (≤56 days of antiretroviral therapy) pediatric subjects are listed in Table 5.
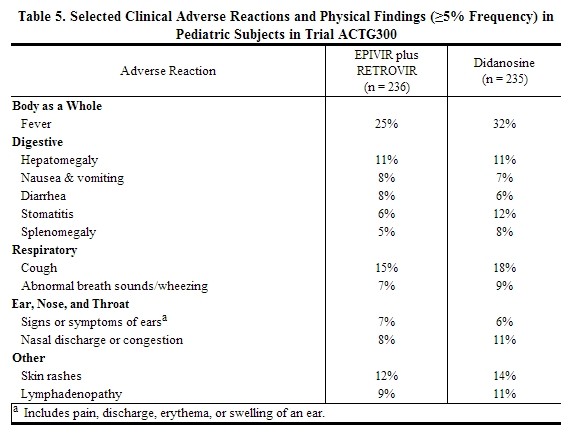 |
Pancreatitis: Pancreatitis, which has been fatal in some cases, has been observed in antiretroviral nucleoside‑experienced pediatric subjects receiving EPIVIR alone or in combination with other antiretroviral agents. In an open‑label dose‑escalation trial (NUCA2002), 14 subjects (14%) developed pancreatitis while receiving monotherapy with EPIVIR. Three of these subjects died of complications of pancreatitis. In a second open‑label trial (NUCA2005), 12 subjects (18%) developed pancreatitis. In Trial ACTG300, pancreatitis was not observed in 236 subjects randomized to EPIVIR plus RETROVIR. Pancreatitis was observed in 1 subject in this trial who received open‑label EPIVIR in combination with RETROVIR and ritonavir following discontinuation of didanosine monotherapy [see Warnings and Precautions (5.5)].
Paresthesias and Peripheral Neuropathies: Paresthesias and peripheral neuropathies were reported in 15 subjects (15%) in Trial NUCA2002, 6 subjects (9%) in Trial NUCA2005, and 2 subjects (<1%) in Trial ACTG300.
Selected laboratory abnormalities experienced by therapy‑naive (56 days of antiretroviral therapy) pediatric subjects are listed in Table 6.
 |
Neonates - Clinical Trials in HIV-1: Limited short-term safety information is available from 2 small, uncontrolled trials in South Africa in neonates receiving lamivudine with or without zidovudine for the first week of life following maternal treatment starting at Week 38 or 36 of gestation [see Clinical Pharmacology (12.3)]. Selected adverse reactions reported in these neonates included increased liver function tests, anemia, diarrhea, electrolyte disturbances, hypoglycemia, jaundice and hepatomegaly, rash, respiratory infections, and sepsis; 3 neonates died (1 from gastroenteritis with acidosis and convulsions, 1 from traumatic injury, and 1 from unknown causes). Two other nonfatal gastroenteritis or diarrhea cases were reported, including 1 with convulsions; 1 infant had transient renal insufficiency associated with dehydration. The absence of control groups limits assessments of causality, but it should be assumed that perinatally exposed infants may be at risk for adverse reactions comparable to those reported in pediatric and adult HIV-1-infected patients treated with lamivudine-containing combination regimens. Long-term effects of in utero and infant lamivudine exposure are not known.
Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been reported during postmarketing use of EPIVIR. Because these reactions are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to lamivudine.
Body as a Whole: Redistribution/accumulation of body fat [see Warnings and Precautions (5.7)].
Endocrine and Metabolic: Hyperglycemia.
General: Weakness.
Hemic and Lymphatic: Anemia (including pure red cell aplasia and severe anemias progressing on therapy).
Hepatic and Pancreatic: Lactic acidosis and hepatic steatosis, posttreatment exacerbation of hepatitis B [see Boxed Warning, Warnings and Precautions (5.1, 5.2)].
Hypersensitivity: Anaphylaxis, urticaria.
Musculoskeletal: Muscle weakness, CPK elevation, rhabdomyolysis.
References
- ↑ "EPIVIR (LAMIVUDINE) TABLET, FILM COATED EPIVIR (LAMIVUDINE) SOLUTION [VIIV HEALTHCARE COMPANY]". Retrieved 9 January 2014.
Adapted from the FDA Package Insert.