Idoxuridine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Idoxuridine is a Antiviral ophthalmologic Agent that is FDA approved for the treatment of keratitis caused by the virus of herpes simplex. Common adverse reactions include Acute ocular irritation including burning, corneal stippling, vascularization, and clouding.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- For the treatment of keratitis caused by the virus of herpes simplex.
Dosage
- Instill one drop in the affected eye(s) every hour. In acute herpes, dosage may be tapered through every two hours to four times daily prior to discontinuation (treatment should be continued for at least seven days).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Idoxuridine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Idoxuridine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Idoxuridine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Idoxuridine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Idoxuridine in pediatric patients.
Contraindications
- Hypersensitivity to the active ingredient or any other component of this drug.
Warnings
Precautions
- For topical use only. Do not exceed the frequency or duration of recommended dosage. The incidence of some adverse reactions increases with prolonged use. Idoxuridine is not effective in corneal inflammations following herpes simplex keratitis in which the virus is not present. Some strains of herpes simplex appear resistant to the action of Idoxuridine.
USAGE IN PREGNANCY
- Idoxuridine should be administered with caution in pregnancy or in women of childbearing potential. Idoxuridine has been reported to cross the placental barrier and to produce fetal malformations in rabbits when administered topically to the eyes of pregnant females in doses similar to those used clinically. Idoxuridine has also been reported to produce fetal malformations in the rat after intraperitoneal and oral administration and in the mouse after subcutaneous administration. Nursing should not be performed while a patient is undergoing IDU treatment as the drug and metabolites may be excreted in human milk.
MUTAGENIC POTENTIAL
- Idoxuridine has been reported to cause chromosome aberrations in mice and to be mutagenic in mammalian cells in culture (e.g., diploid human lymphoblasts and mouse lymphoma cells). Drosophila melanogaster and in a host-mediated assay system utilizing mammalian cells.
ONCOGENIC POTENTIAL
- The studies performed to date on idoxuridine are inadequate for assessment of carcinogenicity. This cytotoxic drug should be regarded as being potentially carcinogenic. It can inhibit DNA synthesis or function and is incorporated into the DNA of mammalian cells as well as into this genome of DNA viruses. Indoxuridine has been reported to induce RNA tumor virus (type C particles) production from virus negative mouse cells. The degree of oncogenic activity of idoxuridine induced oncornaviruses has not been documented. However, several idoxuridine activated oncornaviruses have caused in vitro cell transformation and induction of specific neoplasms (lymphatic leukemias and carcinomas) upon inoculation into syngenic mice.
Adverse Reactions
Clinical Trials Experience
- Acute ocular irritation including burning, corneal stippling, vascularization, and clouding has been reported. Following prolonged use of idoxuridine, ocular irritation characterized by follicular conjunctivitis, blepharitis with punctal swelling, bulbar conjunctival hyperemia, and corneal epithelial staining has also been reported. In either case, the drug should be discontinued.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Idoxuridine in the drug label.
Drug Interactions
There is limited information regarding Idoxuridine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Idoxuridine should be administered with caution in pregnancy or in women of childbearing potential. Idoxuridine has been reported to cross the placental barrier and to produce fetal malformations in rabbits when administered topically to the eyes of pregnant females in doses similar to those used clinically. Idoxuridine has also been reported to produce fetal malformations in the rat after intraperitoneal and oral administration and in the mouse after subcutaneous administration
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Idoxuridine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Idoxuridine during labor and delivery.
Nursing Mothers
- Nursing should not be performed while a patient is undergoing IDU treatment as the drug and metabolites may be excreted in human milk.
Pediatric Use
There is no FDA guidance on the use of Idoxuridine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Idoxuridine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Idoxuridine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Idoxuridine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Idoxuridine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Idoxuridine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Idoxuridine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Idoxuridine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical ophthalmic solution
Monitoring
There is limited information regarding Monitoring of Idoxuridine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Idoxuridine in the drug label.
Overdosage
There is limited information regarding Overdose of Idoxuridine in the drug label.
Pharmacology
| |
Idoxuridine
| |
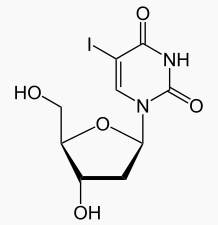
| Systematic (IUPAC) name | |
| 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodo-1,2,3,4-tetrahydropyrimidine-2,4-dione | |
| Identifiers | |
| CAS number | |
| ATC code | D06 J05AB02 (WHO), S01AD01 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 354.099 g/mol |
| SMILES | & |
| Synonyms | Iododeoxyuridine; IUdR |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) B1 (topical), B3 (ophthalmologic) [AU] |
| Legal status | |
| Routes | topically |
Mechanism of Action
- Herpes simplex virus utilizes thymidine in the synthesis of deoxyribonucleic acid (DNA), a metabolite necessary for reproduction. Idoxuridine is identical in chemical structure to thymidine except that the 5-methyl group is replaced by iodine. When idoxuridine is substituted for thymidine in DNA, the cell is unable to utilize the DNA and reproduction ceases.
Structure
- Dendrid® (idoxuridine) is an antiviral chemotherapeutic agent prepared in a sterile buffered isotonic solution. The active ingredient is represented by the chemical structure:
Established name:
Idoxuridine
Chemical name:
Uridine, 2’-deoxy-5-iodo-
Each ml contains: Active: Idoxuridine 0.1%. Preservative: Benzalkonium Chloride 0.01%. Inactive: Boric Acid, Edetate Disodium, Sodium Hydroxide and/or Hydrochloric Acid (to adjust pH), Purified Water. DM-01
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Idoxuridine in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Idoxuridine in the drug label.
Nonclinical Toxicology
MUTAGENIC POTENTIAL
- Idoxuridine has been reported to cause chromosome aberrations in mice and to be mutagenic in mammalian cells in culture (e.g., diploid human lymphoblasts and mouse lymphoma cells). Drosophila melanogaster and in a host-mediated assay system utilizing mammalian cells.
Clinical Studies
There is limited information regarding Clinical Studies of Idoxuridine in the drug label.
How Supplied
- In a 15 ml plastic Drop-Tainer® dispenser.
NDC 0065-0029-15
Storage
- Between 36°-80°F. Protect from light.
Images
Drug Images
{{#ask: Page Name::Idoxuridine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Ingredients and Appearance
{{#ask: Label Page::Idoxuridine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Idoxuridine in the drug label.
Precautions with Alcohol
- Alcohol-Idoxuridine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DENDRID®[1]
Look-Alike Drug Names
There is limited information regarding Idoxuridine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.

