Erythromycin (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Erythromycin (ophthalmic) is a macrolide and antibiotic that is FDA approved for the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by organisms susceptible to erythromycin. For prophylaxis of ophthalmia neonatorum due to N. gonorrhoeae or C. trachomatis. Common adverse reactions include ocular irritations, redness, and hypersensitivity reactions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- For the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by organisms susceptible to erythromycin.
- For prophylaxis of ophthalmia neonatorum due to N. gonorrhoeae or C. trachomatis.
- The effectiveness of erythromycin in the prevention of ophthalmia caused by penicillinase-producing N.gonorrhoeae is not established.
- For infants born to mothers with clinically apparent gonorrhea, intravenous or intramuscular injections of aqueous crystalline penicillin G should be given; a single dose of 50,000 units for term infants or 20,000 units for infants of low birth weight. Topical prophylaxis alone is inadequate for these infants.
Dosing Information
- In the treatment of superficial ocular infections, a ribbon approximately 1 cm in length of Erythromycin Opthalmic Ointment should be applied directly to the infected structure up to 6 times daily, depending on the severity of the infection.
- For prophylaxis of neonatal gonococcal or chlamydial conjunctivitis, a ribbon of ointment approximately 1 cm in length should be instilled into each lower conjunctival sac. The ointment should not be flushed from the eye following instillation. A new tube should be used for each infant.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Erythromycin (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Erythromycin (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Erythromycin (ophthalmic) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Erythromycin (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Erythromycin (ophthalmic) in pediatric patients.
Contraindications
- This drug is contraindicated in patients with a history of hypersensitivity to erythromycin.
Warnings
PRECAUTIONS:=
General:
- The use of antimicrobial agents may be associated with the overgrowth of nonsusceptible organisms including fungi; in such a case, antibiotic administration should be stopped and appropriate measures taken.
Adverse Reactions
Clinical Trials Experience
- The most frequently reported adverse reactions are minor ocular irritations, redness, and hypersensitivity reactions.
Postmarketing Experience
There is limited information regarding Erythromycin (ophthalmic) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Erythromycin (ophthalmic) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in rats, mice, and rabbits using erythromycin and its various salts and esters, at doses that were several multiples of the usual human dose. No evidence of harm to the fetus that appeared related to erythromycin was reported in these studies. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, the erythromycins should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Erythromycin (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Erythromycin (ophthalmic) during labor and delivery.
Nursing Mothers
- Caution should be exercised when erythromycin is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Erythromycin (ophthalmic) in pediatric settings.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Erythromycin (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Erythromycin (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Erythromycin (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Erythromycin (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Erythromycin (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Erythromycin (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Erythromycin (ophthalmic) Administration in the drug label.
Monitoring
There is limited information regarding Erythromycin (ophthalmic) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Erythromycin (ophthalmic) and IV administrations.
Overdosage
There is limited information regarding Erythromycin (ophthalmic) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Erythromycin acts by inhibition of protein synthesis by binding 50 S ribosomal subunits of susceptible organisms. It does not affect nucleic acid synthesis.
Structure
- Erythromycin Delayed-release Capsules contain enteric-coated pellets of erythromycin base for oral administration.
- Each Erythromycin Delayed-release Capsule contains 250 mg of erythromycin base.
- Inactive Ingredients
- Cellulosic polymers, citrate ester, D&C Red No. 30, D&C Yellow No. 10, magnesium stearate and povidone. The capsule shell contains FD&C Blue No. 1, FD&C Red No. 3, gelatin, and titanium dioxide.
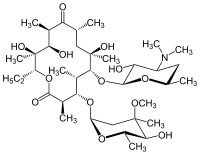
- Erythromycin is produced by a strain of Saccharopolyspora erythraea (formerly Streptomyces erythraeus) and belongs to the macrolide group of antibiotics. It is basic and readily forms salts with acids but it is the base which is microbiologically active. Erythromycin base is (3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6- Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl) oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione.
Pharmacodynamics
Microbiology:
- Erythromycin inhibits protein synthesis without affecting nucleic acid synthesis. Erythromycin is usually active against the following organisms in vitro and in clinical infections:
- Streptococcus pyogenes (group A β-hemolytic)
- Alpha-hemolytic streptococci (viridans group)
- Staphylococcus aureus, including penicillinase-producing strains (methicillin-resistant staphylococci are uniformly resistant to erythromycin)
- Streptococcus pneumoniae
- Mycoplasma pneumoniae (Eaton Agent, PPLO)
- Haemophilus influenzae (not all strains of this organism are susceptible at the erythromycin concentrations ordinarily achieved)
- Treponema pallidum
- Corynebacterium diphtheriae
- Neisseria gonorrhoeae
- Chlamydia trachomatis
Pharmacokinetics
There is limited information regarding Erythromycin (ophthalmic) Pharmacokinetics in the drug label.
Nonclinical Toxicology
- Two year oral studies conducted in rats with erythromycin did not provide evidence of tumorigenicity. Mutagenicity studies have not been conducted. No evidence of impaired fertility that appeared related to erythromycin was reported in animal studies.
Clinical Studies
There is limited information regarding Erythromycin (ophthalmic) Clinical Studies in the drug label.
How Supplied
Sterile Erythromycin Ophthalmic Ointment USP, 0.5% is available as follows:
3.5 g (1/8 oz) sterile tamper-resistant tube
(NDC 17478-824-35)
Carton of fifty (50) Unit Dose 1 g tube
(NDC 17478-824-01)
Avoid excessive heat.
Protect from freezing.
PREMIERPro™Rx Manufactured by: Akorn Inc Lake Forest, IL 60045
PREMIERPro™Rx is a trademark of Premier Inc., used under license.
PERT00N Rev. 12/12
Storage
- Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Erythromycin (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Principal Display Panel Text for Container Label:
NDC 17478-824-35 Premier Logo
ERYTHROMYCIN OPHTHALMIC
OINTMENT USP, 0.5%
Rx only Sterile
Net Wt. 3.5 g (1/8 oz.)

Principal Display Panel Text for Carton Label:
NDC 17478-824-35
ERYTHROMYCIN OPHTHALMIC
OINTMENT USP, 0.5%
Sterile
Net Wt 3.5 g (1/8 oz.)
Rx only Premier Logo

{{#ask: Label Page::Erythromycin (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Avoid contaminating the applicator tip with material from the eye, fingers, or other source.
Precautions with Alcohol
Alcohol-Erythromycin (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Erythromycin (ophthalmic) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Erythromycin (ophthalmic) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.