Chloramphenicol (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning
See full prescribing information for complete Boxed Warning.
* Bone marrow hypoplasia, including aplastic anemia and death has been reported following local application of chloramphenicol. Chloramphenicol should not be used when less potentially dangerous agents would be expected to provide effective treatment.
|
Overview
Chloramphenicol (ophthalmic) is an antibiotic that is FDA approved for the treatment of ocular infections involving the conjunctiva and/or cornea caused by chloramphenicol-susceptible organisms.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include optic atrophy and blood dyscrasias.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Chloramphenicol should be used only in those serious infections for which less potentially dangerous drugs are ineffective or contraindicated. Bacteriological studies should be performed to determine the causative organisms and their sensitivity to chloramphenicol. For treatment of ocular infections involving the conjunctiva and/or cornea caused by chloramphenicol-susceptible organisms.
- Chloramphenicol has a wide spectrum of antimicrobial activity and is effective against many gram negative and gram positive bacteria, including the following common eye pathogens:
- Streptococci, including Streptococcus pneumoniae
- Klebsiella/Enterobacter species
- Neisseria species
- Moraxella lacunata (Morax-Axenfeld bacillus)
- The product does not provide adequate coverage against:
Dosage
- Solution: In severe disease, instill two drops on the eye(s) hourly until improvement, following which treatment should be diminished prior to discontinuation. In mild disease, drops may be used four times daily. Ointment: Apply a small amount to the lower conjunctival sac(s) at bedtime as a supplement to the drops.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chloramphenicol (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chloramphenicol (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Chloramphenicol (ophthalmic) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chloramphenicol (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chloramphenicol (ophthalmic) in pediatric patients.
Contraindications
- This product is contraindicated in those persons who have shown hypersensitivity to any of its components.
Warnings
|
Warning
See full prescribing information for complete Boxed Warning.
* Bone marrow hypoplasia, including aplastic anemia and death has been reported following local application of chloramphenicol. Chloramphenicol should not be used when less potentially dangerous agents would be expected to provide effective treatment.
|
- Ophthalmic ointment may retard corneal wound healing.
Adverse Reactions
Clinical Trials Experience
- Blood dyscrasias may be associated with the systemic use of chloramphenicol. One case of bone-marrow hypoplasia following the prolonged (23 month) topical use of chloramphenicol ophthalmic solution has been reported.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Chloramphenicol (ophthalmic) in the drug label.
Drug Interactions
There is limited information regarding Chloramphenicol (ophthalmic) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Chloramphenicol (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Chloramphenicol (ophthalmic) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Chloramphenicol (ophthalmic) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Chloramphenicol (ophthalmic) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Chloramphenicol (ophthalmic) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Chloramphenicol (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Chloramphenicol (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Chloramphenicol (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Chloramphenicol (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Chloramphenicol (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Chloramphenicol (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic
Monitoring
There is limited information regarding Monitoring of Chloramphenicol (ophthalmic) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Chloramphenicol (ophthalmic) in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Chloramphenicol (ophthalmic) in the drug label.
Pharmacology
Mechanism of Action
It is primarily bacteriostatic and acts by inhibition of protein synthesis by interfering with the transfer of activated amino acids from soluble RNA to ribosomes.
Structure
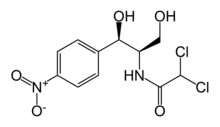
- ECONOCHLOR® (Chloramphenicol) is a sterile topical ophthalmic antibacterial prepared in solution and ointment forms. The active ingredient is represented by the chemical structure:
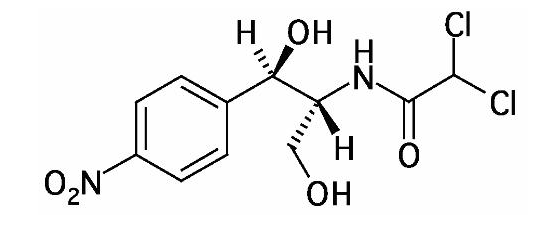
- Established name:
- Chloramphenicol
- Chemical name:
- Acetamide, 2,2-dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2- (4-nitrophenyl) ethyl]-, [R-(R*,R)]-
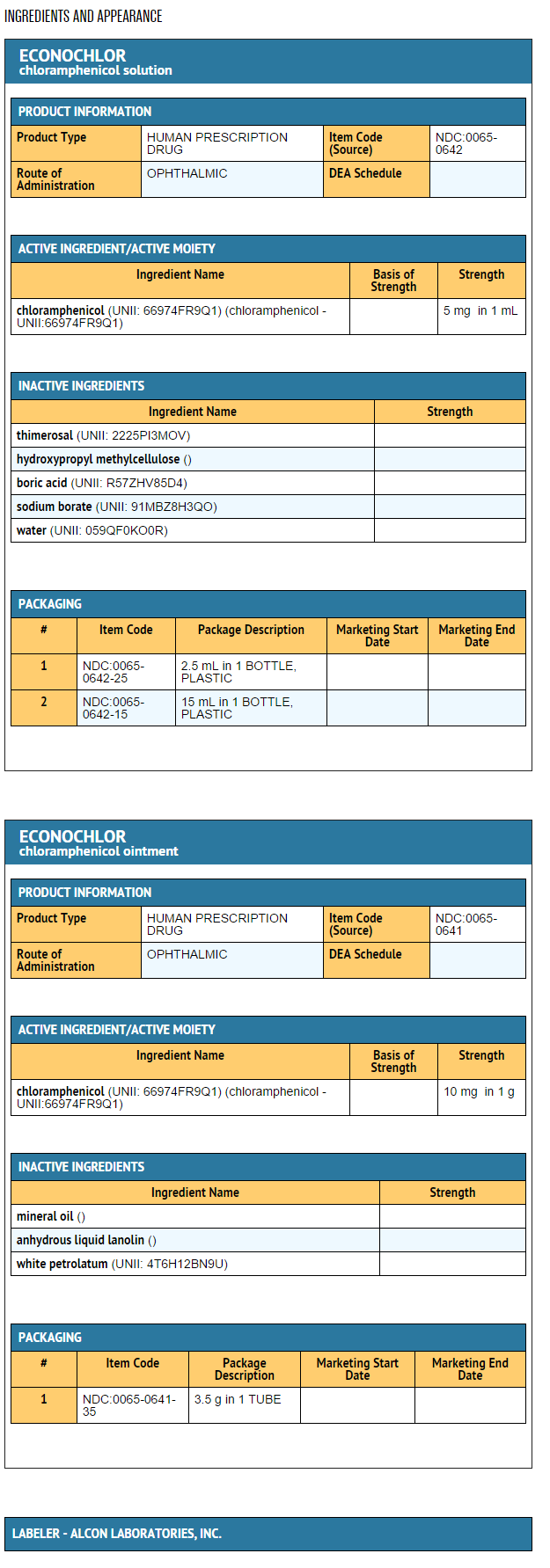
- Each ml of solution contains: Active: Chloramphenicol 0.5% (5mg/ml). Preservative: Thimerosal 0.01%. Vehicle: Hydroxypropyl Methylcellulose: Inactive: Boric Acid, Sodium Borate (to adjust pH), Purified Water.
- DM-00
- Each gram of ointment contains: Active: Chloramphenicol 1.0% (10 mg/g). Inactive: Mineral Oil, Anhydrous Liquid Lanolin, White Petrolatum.
- DM-00
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Chloramphenicol (ophthalmic) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Chloramphenicol (ophthalmic) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Chloramphenicol (ophthalmic) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Chloramphenicol (ophthalmic) in the drug label.
How Supplied
- Solution in 5ml (2.5ml fill) and 15ml plastic Drop-Tainer® dispensers. Ointment in 3.5 gram ophthalmic tube. Rx only.
- 2.5ml NDC 0065-0642-25
- 15ml NDC 0065-0642-15
- 3.5 g NDC 0065-0641-35
Storage
- Solution: Refrigerate until dispensed. Ointment: Store between 46° and 80°F.
Images
Drug Images
{{#ask: Page Name::Chloramphenicol (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Chloramphenicol (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Chloramphenicol (ophthalmic) in the drug label.
Precautions with Alcohol
- Alcohol-Chloramphenicol (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ECONOCHLOR ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "chloramphenicol ointment".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Chloramphenicol (ophthalmic)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Chloramphenicol (ophthalmic) |Label Name=Chloramphenicol (ophthalmic)11.png
}}
{{#subobject:
|Label Page=Chloramphenicol (ophthalmic) |Label Name=Chloramphenicol (ophthalmic)11.png
}}