Anidulafungin clinical pharmacology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Clinical Pharmacology
Mechanism of Action
Anidulafungin is an anti-fungal drug.
Pharmacokinetics
General Pharmacokinetic Characteristics
The pharmacokinetics of anidulafungin following intravenous (IV) administration have been characterized in healthy subjects, special populations and patients. Systemic exposures of anidulafungin are dose-proportional and have low intersubject variability (coefficient of variation <25%) as shown in Table 4. The steady state was achieved on the first day after a loading dose (twice the daily maintenance dose) and the estimated plasma accumulation factor at steady state is approximately 2.
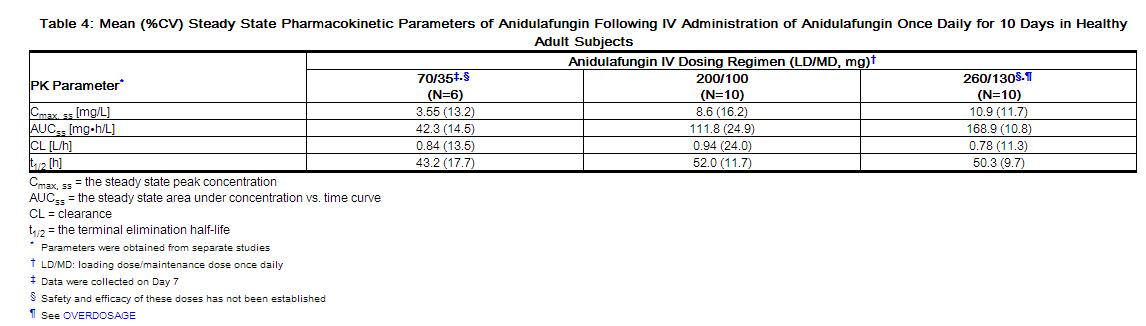 |
The clearance of anidulafungin is about 1 L/h and anidulafungin has a terminal elimination half-life of 40–50 hours.
Distribution
The pharmacokinetics of anidulafungin following IV administration are characterized by a short distribution half-life (0.5–1 hour) and a volume of distribution of 30–50 L that is similar to total body fluid volume. Anidulafungin is extensively bound (>99%) to human plasma proteins.
Metabolism
Hepatic metabolism of anidulafungin has not been observed. Anidulafungin is not a clinically relevant substrate, inducer, or inhibitor of cytochrome P450 (CYP450) isoenzymes. It is unlikely that anidulafungin will have clinically relevant effects on the metabolism of drugs metabolized by CYP450 isoenzymes.
Anidulafungin undergoes slow chemical degradation at physiologic temperature and pH to a ring-opened peptide that lacks antifungal activity. The in vitrodegradation half-life of anidulafungin under physiologic conditions is about 24 hours. In vivo, the ring-opened product is subsequently converted to peptidic degradants and eliminated.
Excretion
In a single-dose clinical study, radiolabeled (14C) anidulafungin was administered to healthy subjects. Approximately 30% of the administered radioactive dose was eliminated in the feces over 9 days, of which less than 10% was intact drug. Less than 1% of the administered radioactive dose was excreted in the urine. Anidulafungin concentrations fell below the lower limits of quantitation 6 days post-dose. Negligible amounts of drug-derived radioactivity were recovered in blood, urine, and feces 8 weeks post-dose.
Specific Populations
- Patients with fungal infections
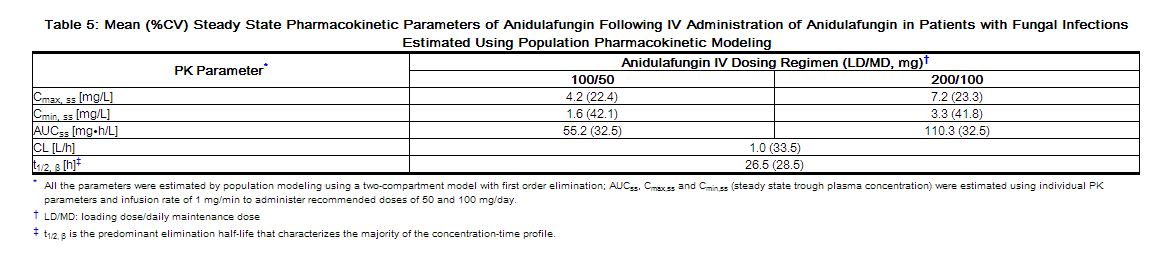
Population pharmacokinetic analyses from four clinical trials including 107 male and 118 female patients with fungal infections showed that the pharmacokinetic parameters of anidulafungin are not affected by age, race, or the presence of concomitant medications which are known metabolic substrates, inhibitors or inducers.
The pharmacokinetics of anidulafungin in patients with fungal infections are similar to those observed in healthy subjects. The pharmacokinetic parameters of anidulafungin estimated using population pharmacokinetic modeling following IV administration of a maintenance dose of 50 mg/day or 100 mg/day (following a loading dose) are presented in Table 5.
 |
- Gender
Dosage adjustments are not required based on gender. Plasma concentrations of anidulafungin in healthy men and women were similar. In multiple-dose patient studies, drug clearance was slightly faster (approximately 22%) in men.
- Geriatric
Dosage adjustments are not required for geriatric patients. The population pharmacokinetic analysis showed that median clearance differed slightly between the elderly group (patients ≥65, median CL=1.07 L/h) and the non-elderly group (patients <65, median CL=1.22 L/h) and the range of clearance was similar.
- Race
Dosage adjustments are not required based on race. Anidulafungin pharmacokinetics were similar among Whites, Blacks, Asians, and Hispanics.
- HIV Status
Dosage adjustments are not required based on HIV status, irrespective of concomitant anti-retroviral therapy.
- Hepatic Insufficiency
Dosage adjustments are not required on the basis of mild, moderate or severe hepatic insufficiency. Anidulafungin is not hepatically metabolized. Anidulafungin pharmacokinetics were examined in subjects with Child-Pugh class A, B or C hepatic insufficiency. Anidulafungin concentrations were not increased in subjects with any degree of hepatic insufficiency. Though a slight decrease in AUC was observed in patients with Child-Pugh C hepatic insufficiency, it was within the range of population estimates noted for healthy subjects.
- Renal Insufficiency
Dosage adjustments are not required for patients with any degree of renal insufficiency including those on hemodialysis. Anidulafungin has negligible renal clearance. In a clinical study of subjects with mild, moderate, severe or end stage (dialysis-dependent) renal insufficiency, anidulafungin pharmacokinetics were similar to those observed in subjects with normal renal function. Anidulafungin is not dialyzable and may be administered without regard to the timing of hemodialysis.
- Pediatric
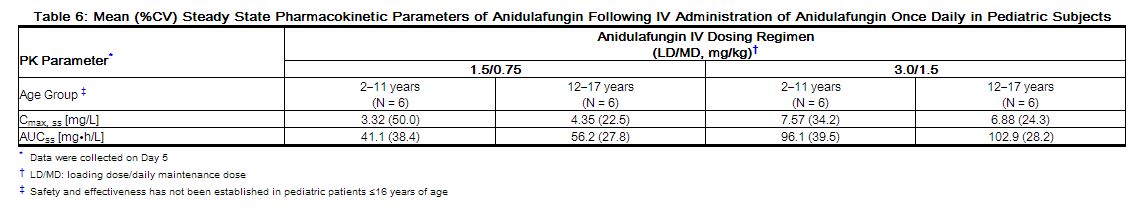
The pharmacokinetics of anidulafungin after daily doses were investigated in immunocompromised pediatric (2 through 11 years) and adolescent (12 through 17 years) patients with neutropenia. The steady state was achieved on the first day after administration of the loading dose (twice the maintenance dose), and the Cmax and AUCss increased in a dose-proportional manner. Concentrations and exposures following administration of maintenance doses of 0.75 and 1.5 mg/kg/day in this population were similar to those observed in adults following maintenance doses of 50 and 100 mg/day, respectively (as shown in Table 6).
 |
Clinical Studies
Candidemia and Other Candida Infections (Intra-abdominal Abscess and Peritonitis)
The safety and efficacy of ERAXIS were evaluated in a Phase 3, randomized, double-blind study of patients with candidemia and/or other forms of invasive candidiasis. Patients were randomized to receive once daily IV ERAXIS (200 mg loading dose followed by 100 mg maintenance dose) or IV fluconazole (800 mg loading dose followed by 400 mg maintenance dose). Patients were stratified by APACHE II score (≤20 and >20) and the presence or absence of neutropenia. Patients with Candida endocarditis, osteomyelitis or meningitis, or those with infection due to C. krusei, were excluded from the study. Treatment was administered for at least 14 and not more than 42 days. Patients in both study arms were permitted to switch to oral fluconazole after at least 10 days of intravenous therapy, provided that they were able to tolerate oral medication, were afebrile for at least 24 hours, and the last blood cultures were negative for Candida species.
Patients who received at least one dose of study medication and who had a positive culture for Candida species from a normally sterile site before entry into the study (modified intent-to-treat [MITT] population) were included in the analysis of global response at the end of IV therapy. A successful global response required clinical cure or improvement (significant, but incomplete resolution of signs and symptoms of the Candida infection and no additional antifungal treatment), and documented or presumed microbiological eradication. Patients with an indeterminate outcome were analyzed as failures in this population.
Two hundred and fifty-six patients in the intent-to-treat (ITT) population were randomized and received at least one dose of study medication. In ERAXIS-treated patients, the age range was 16–89 years, the gender distribution was 50% male and 50% female, and the race distribution was 71% White, 20% Black/African American, 7% Hispanic, 2% other races. The median duration of IV therapy was 14 and 11 days in the ERAXIS and fluconazole arms, respectively. For those who received oral fluconazole, the median duration of oral therapy was 7 days for the ERAXIS arm and 5 days for the fluconazole arm.
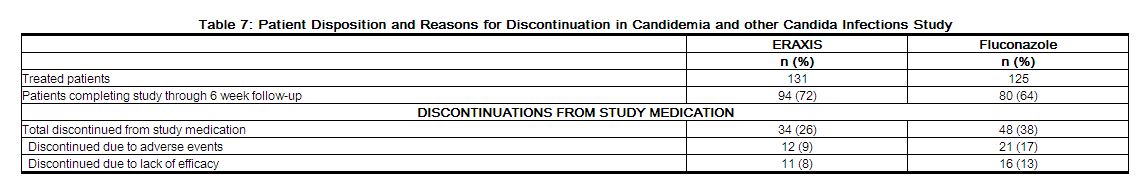
Patient disposition is presented in Table 7.
 |
Two hundred and forty-five patients (127 ERAXIS, 118 fluconazole) met the criteria for inclusion in the MITT population. Of these, 219 patients (116 ERAXIS, 103 fluconazole) had candidemia only. Risk factors for candidemia among patients in both treatment arms in this study were: presence of a central venous catheter (78%), receipt of broad-spectrum antibiotics (69%), recent surgery (42%), recent hyperalimentation (25%), and underlying malignancy (22%). The most frequent species isolated at baseline was C. albicans (62%), followed by C. glabrata (20%), C. parapsilosis (12%) and C. tropicalis (11%). The majority (97%) of patients were non-neutropenic (ANC >500) and 81% had APACHE II scores less than or equal to 20.
Global success rates in patients with candidemia and other Candida infections are summarized in Table 8.
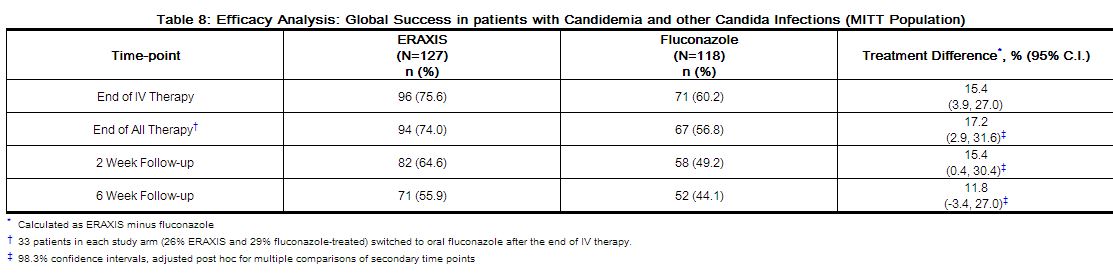 |
Table 9 presents global response by patients with candidemia or multiple sites of Candida infection and mortality data for the MITT population.
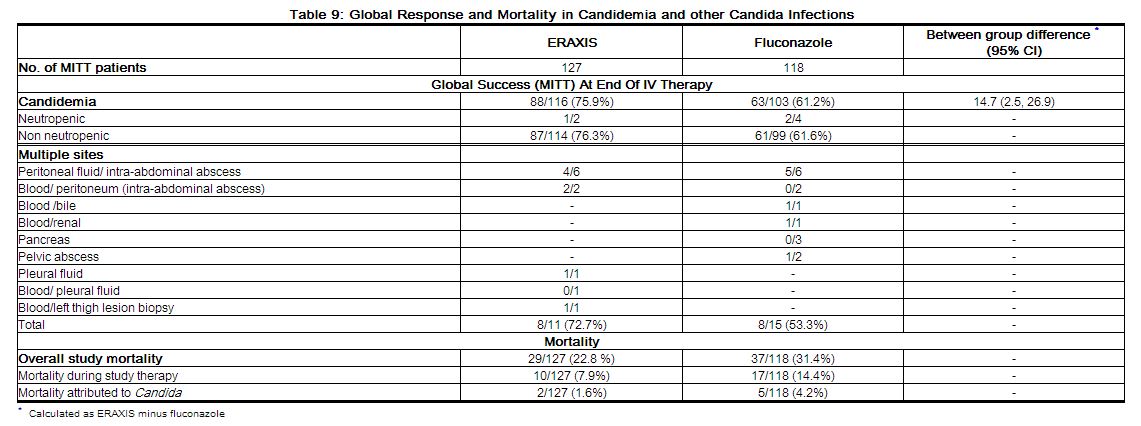 |
Esophageal Candidiasis
ERAXIS was evaluated in a double-blind, double-dummy, randomized Phase 3 study. Three hundred patients received ERAXIS (100 mg loading dose IV on Day 1 followed by 50 mg/day IV) and 301 received oral fluconazole (200 mg loading dose on Day 1 followed by 100 mg/day). Treatment duration was 7 days beyond resolution of symptoms for a minimum of 14 and a maximum of 21 days.
Of the 442 patients with culture confirmed esophageal candidiasis, most patients (91%) had C. albicans isolated at the baseline.
Treatment groups were similar in demographic and other baseline characteristics. In ERAXIS-treated patients, the age range was 16–69 years, the gender distribution was 42% male and 58% female, and the race distribution was 15% White, 49% Black/African American, 15% Asian, 0.3 % Hispanic, 21% other races.
In this study, of 280 patients tested, 237 (84.6%) tested HIV positive. In both groups the median time to resolution of symptoms was 5 days and the median duration of therapy was 14 days.
Efficacy was assessed by endoscopic outcome at end of therapy (EOT). Patients were considered clinically evaluable if they received at least 10 days of therapy, had an EOT assessment with a clinical outcome other than 'indeterminate', had an endoscopy at EOT, and did not have any protocol violations prior to the EOT visit that would affect an assessment of efficacy.
An endoscopic success, defined as cure (endoscopic grade of 0 on a 4-point severity scale) or improvement (decrease of one or more grades from baseline), was seen in 225/231 (97.4%) ERAXIS-treated patients and 233/236 (98.7%) fluconazole-treated patients (Table 10). The majority of these patients were endoscopic cures (grade=0). Two weeks after completing therapy, the ERAXIS group had significantly more endoscopically-documented relapses than the fluconazole group, 120/225 (53.3%) vs. 45/233 (19.3%), respectively (Table 10). T10 Clinical success (cure or improvement in clinical symptoms including odynophagia/dysphagia and retrosternal pain) occurred in 229/231 (99.1%) of the ERAXIS-treated patients and 235/236 (99.6%) of the fluconazole-treated patients at the end of therapy. For patients with C. albicans, microbiological success occurred in 142/162 (87.7%) of the ERAXIS-treated group and 157/166 (94.6%) of the fluconazole-treated group at the end of therapy. For patients with Candida species other than C. albicans, success occurred in 10/12 (83.3%) of the ERAXIS-treated group and 14/16 (87.5%) of the fluconazole-treated group.[1]
References
Adapted from the FDA Package Insert.