Von Willebrand factor
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Von Willebrand factor is a plasma derivate that is FDA approved for the treatment of hemophilia A and surgical and/or invasive procedures in adult and pediatric patients with von Willebrand Disease in whom desmopressin (DDAVP) is either ineffective or contraindicated.. Common adverse reactions include edema of face, pruritus, rash, urticaria. nausea, dizziness, headache, paresthesia, pharyngitis, pain, shivering and factor VIII disorder.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hemophilia A: Control and prevention of bleeding episodes
- Dose (units) = body weight (kg) x desired FVIII rise (IU/dL or % of normal) x 0.5 (IU/kg per IU/dL).
- Frequency of intravenous injection of the reconstituted product is determined by the type of bleeding episode and the recommendation of the treating physician.
Von Willebrand Disease: Surgical and/or Invasive Procedure in Adult and Pediatric patients
- Dosage: Pre-operative dose of 60 IU VWF:RCo/kg body weight; subsequent doses of 40-60 IU VWF:RCo/kg body weight at 8-12 hour intervals post-operative as clinically needed.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Von Willebrand factor in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Von Willebrand factor in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Von Willebrand Disease: Surgical and/or Invasive Procedure in Adult and Pediatric patients
- Dosage: Pre-operative dose of 75 IU VWF:RCo/kg body weight; subsequent doses of 50-75 IU VWF:RCo/kg body weight at 8-12 hour intervals post-operative as clinically needed.
Hemophilia A: Control and prevention of bleeding episodes
- Dose (units) = body weight (kg) x desired FVIII rise (IU/dL or % of normal) x 0.5 (IU/kg per IU/dL).
- Frequency of intravenous injection of the reconstituted product is determined by the type of bleeding episode and the recommendation of the treating physician.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Von Willebrand factor in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Von Willebrand factor in pediatric patients.
Contraindications
Alphanate is contraindicated in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product or its components.
Warnings
Anaphylaxis and Severe Hypersensitivity Reactions
Anaphylaxis and severe hypersensitivity reactions are possible. Should symptoms occur, treatment with Alphanate should be discontinued, and emergency treatment should be administered.
Neutralizing Antibodies
Development of procoagulant activity-neutralizing antibodies (inhibitors) has been detected in patients receiving FVIII-containing products. Carefully monitor patients treated with AHF products for the development of FVIII inhibitors by appropriate clinical observations and laboratory tests. No studies have been conducted with Alphanate to evaluate inhibitor formation. Therefore, it is not known whether there are greater, lesser or the same risks of developing inhibitors due to the use of this product than there are with other FVIII preparations. If expected plasma FVIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, an assay that measures FVIII inhibitor concentration should be performed. Patients with these inhibitors may not respond to treatment with Antihemophilic Factor/von Willebrand Factor Complex (Human), or the response may be much less than would otherwise be expected; therefore, larger doses of Antihemophilic Factor/von Willebrand Factor Complex (Human) are often required. The management of bleeding in patients with inhibitors requires careful monitoring, especially if surgical procedures are indicated.mDepending on the level of the inhibitor and/or clinical response, it may be appropriate to use an alternative ‘bypass’ therapeutic agent.
Reports in the literature suggest that patients with Type 3, severe von Willebrand Disease, may develop alloantibodies to von Willebrand factor (VWF) after replacement therapy. The risk of developing alloantibodies in patients with von Willebrand disease due to the use of this product is not known.
Thromboembolic Events
Thromboembolic events have been reported in von Willebrand Disease patients receiving AHF/VWF Complex (Human) replacement therapy, especially in the setting of known risk factors for thrombosis. In addition, endogenous high levels of FVIII have also been associated with thrombosis but no causal relationship has been established. In all VWD patients in situations of high thrombotic risk receiving coagulation factor replacement therapy, caution should be exercised and antithrombotic measures should be considered.
Intravascular Hemolysis
Massive doses of AHF/VWF Complex (Human) have resulted in a few cases of acute hemolytic anemia, increased bleeding tendency or hyperfibrinogenemia as reported in the literature, which subside after cessation of the commercial factor infusion. Alphanate contains blood group specific isoagglutinins and, when large and/or frequent doses are required in patients of blood groups A, B, or AB, the patient should be monitored for signs of intravascular hemolysis and falling hematocrit. Should this condition occur, thus leading to progressive hemolytic anemia, the administration of serologically compatible Type O red blood cells should be considered, the administration of Alphanate should be discontinued, and alternative therapy should be considered.
Vasomotor Reactions
Rapid administration of a FVIII concentrate may result in vasomotor reactions. Alphanate should not be administered at a rate exceeding 10 mL/minute.
Transmissible Infectious Agents
Because Alphanate is made from pooled human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the Creutzfeldt-Jakob Disease (CJD) agent. Stringent procedures designed to reduce the risk of adventitious agent transmission have been employed in the manufacture of this product, from the screening of plasma donors and the collection and testing of plasma, through the application of viral elimination/reduction steps such as solvent detergent and heat treatment in the manufacturing process. Despite these measures, such products can still potentially transmit disease; therefore, the risk of infectious agents cannot be totally eliminated.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Von Willebrand factor Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Von Willebrand factor Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Von Willebrand factor Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Von Willebrand factor in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Von Willebrand factor in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Von Willebrand factor during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Von Willebrand factor in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Von Willebrand factor in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Von Willebrand factor in geriatric settings.
Gender
There is no FDA guidance on the use of Von Willebrand factor with respect to specific gender populations.
Race
There is no FDA guidance on the use of Von Willebrand factor with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Von Willebrand factor in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Von Willebrand factor in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Von Willebrand factor in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Von Willebrand factor in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Von Willebrand factor Administration in the drug label.
Monitoring
There is limited information regarding Von Willebrand factor Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Von Willebrand factor and IV administrations.
Overdosage
There is limited information regarding Von Willebrand factor overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Von Willebrand factor Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Von Willebrand factor Mechanism of Action in the drug label.
Structure
There is limited information regarding Von Willebrand factor Structure in the drug label.
Pharmacodynamics
There is limited information regarding Von Willebrand factor Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Von Willebrand factor Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Von Willebrand factor Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Von Willebrand factor Clinical Studies in the drug label.
How Supplied
There is limited information regarding Von Willebrand factor How Supplied in the drug label.
Storage
There is limited information regarding Von Willebrand factor Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Von Willebrand factor |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Von Willebrand factor |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Von Willebrand factor Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Von Willebrand factor interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Von Willebrand factor Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Von Willebrand factor Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| Von Willebrand factor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
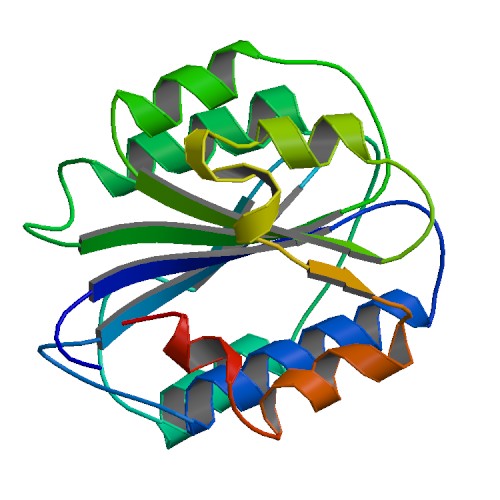 PDB rendering based on 1ao3. | |||||||||||
| Identifiers | |||||||||||
| Symbols | VWF ; F8VWF; VWD | ||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 466 | ||||||||||
| |||||||||||
| RNA expression pattern | |||||||||||
 | |||||||||||
| More reference expression data | |||||||||||
| Orthologs | |||||||||||
| Template:GNF Ortholog box | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | n/a | n/a | |||||||||
| Ensembl | n/a | n/a | |||||||||
| UniProt | n/a | n/a | |||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||
| RefSeq (protein) | n/a | n/a | |||||||||
| Location (UCSC) | n/a | n/a | |||||||||
| PubMed search | n/a | n/a | |||||||||
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Von Willebrand factor is a blood glycoprotein involved in hemostasis. It is deficient or defective in von Willebrand disease and is involved in a large number of other diseases, including thrombotic thrombocytopenic purpura, Heyde's syndrome, and possibly hemolytic-uremic syndrome.[1]
Biochemistry
Synthesis
vWF is a large multimeric glycoprotein present in blood plasma and produced constitutively in endothelium (in the Weibel-Palade bodies), megakaryocytes (α-granules of platelets), and subendothelial connective tissue.[1]
Structure
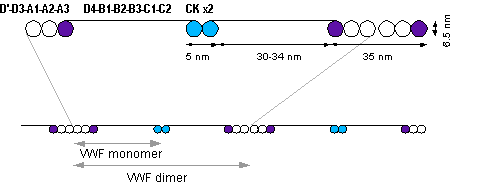
The basic vWF monomer is a 2050 amino acid protein. Every monomer contains a number of specific domains with a specific function; elements of note are:[1]
- the D'/D3 domain, which binds to Factor VIII
- the A1 domain, which binds to:
- the A3 domain, which binds to collagen
- the C1 domain, in which the RGD domain binds to platelet integrin αIIbβ3 when this is activated
- the "cysteine knot" domain (at the C-terminal end of the protein), which vWF shares with platelet-derived growth factor (PDGF), transforming growth factor-β (TGFβ) and β-human chorionic gonadotropin (βHCG, of pregnancy test fame).
Monomers are subsequently N-glycosylated, arranged into dimers in the endoplasmic reticulum and into multimers in the Golgi apparatus by crosslinking of cysteine residues via disulfide bonds. With respect to the glycosylation, vWF is one of the few proteins that carry ABO blood group system antigens.[1]
Multimers of vWF can be extremely large, >20,000 kDa, and consist of over 80 subunits of 250 kDa each. Only the large multimers are functional. Some cleavage products that result from vWF production are also secreted but probably serve no function.[1]
Function
Von Willebrand factor is not an enzyme and therefore has no catalytic activity. Its primary function is binding to other proteins, particularly Factor VIII and it is important in platelet adhesion to wound sites.[1]
vWF binds to a number of cells and molecules. The most important ones are:[1]
- Factor VIII is bound to vWF while inactive in circulation; Factor VIII degrades rapidly when not bound to vWF. Factor VIII is released from vWF by the action of thrombin.
- vWF binds to collagen, e.g., when it is exposed in endothelial cells due to damage occurring to the blood vessel.
- vWF binds to platelet gpIb when it forms a complex with gpIX and gpV; this binding occurs under all circumstances, but is most efficient under high shear stress (i.e., rapid blood flow in narrow blood vessels, see below).
- vWF binds to other platelet receptors when they are activated, e.g., by thrombin (i.e., when coagulation has been stimulated).
vWF appears to play a major role in blood coagulation. vWF deficiency or dysfunction (von Willebrand disease) therefore leads to a bleeding tendency, which is most apparent in tissues having high blood flow shear in narrow vessels. From studies it appears that vWF uncoils under these circumstances, decelerating passing platelets.[1]
Catabolism
The biological breakdown (catabolism) of vWF is largely mediated by a protein cryptically termed ADAMTS13 (acronym of "a disintegrin-like and metalloprotease with thrombospondin type 1 motif no. 13"). It is a metalloproteinase which cleaves vWF between tyrosine at position 842 and methionine at position 843 (or 1605-1606 of the gene) in the A2 domain. This breaks down the multimers into smaller units, which are degraded by other peptidases.[2]
Role in disease
Hereditary or acquired defects of vWF lead to von Willebrand disease (vWD), a bleeding diathesis of the skin and mucous membranes, causing nosebleeds, menorrhagia, and gastrointestinal bleeding. The point at which the mutation occurs determines the severity of the bleeding diathesis. There are three types (I, II and III), and type II is further divided in several subtypes. Treatment depends on the nature of the abnormality and the severity of the symptoms.[3] Most cases of vWD are hereditary, but abnormalities to vWF may be acquired; aortic valve stenosis, for instance, has been linked to vWD type IIA, causing gastrointestinal bleeding - an association known as Heyde's syndrome.[4]
In thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS), ADAMTS13 either is deficient or has been inhibited by antibodies directed at the enzyme. This leads to decreased breakdown of the ultra-large multimers of vWF and microangiopathic hemolytic anemia with deposition of fibrin and platelets in small vessels, and capillary necrosis. In TTP, the organ most obviously affected is the brain; in HUS, the kidney.[5]
Higher levels of vWF are more common among people that have had ischaemic stroke (from blood-clotting) for the first time. Occurrence is not affected by ADAMTS13, and the only significant genetic factor is the person's blood group.[6]
History
vWF is named after Dr. Erik von Willebrand (1870-1949), a Finnish doctor who in 1924 first described a hereditary bleeding disorder in families from the Åland islands, who had a tendency for cutaneous and mucosal bleeding, including menorrhagia. Although von Willebrand could not identify the definite cause, he distinguished von Willebrand disease (vWD) from haemophilia and other forms of bleeding diathesis.[7]
In the 1950s, vWD was shown to be caused by a plasma factor deficiency (instead of being caused by platelet disorders), and, in the 1970s, the vWF protein was purified.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Sadler JE (1998). "Biochemistry and genetics of von Willebrand factor". Annu. Rev. Biochem. 67: 395–424. doi:10.1146/annurev.biochem.67.1.395. PMID 9759493.
- ↑ Levy GG, Motto DG, Ginsburg D (2005). "ADAMTS13 turns 3". Blood. 106 (1): 11–7. doi:10.1182/blood-2004-10-4097. PMID 15774620.
- ↑ Sadler JE, Budde U, Eikenboom JC; et al. (2006). "Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor". J. Thromb. Haemost. 4 (10): 2103–14. doi:10.1111/j.1538-7836.2006.02146.x. PMID 16889557.
- ↑ Vincentelli A, Susen S, Le Tourneau T; et al. (2003). "Acquired von Willebrand syndrome in aortic stenosis". N. Engl. J. Med. 349 (4): 343–9. doi:10.1056/NEJMoa022831. PMID 12878741.
- ↑ Moake JL (2004). "von Willebrand factor, ADAMTS-13, and thrombotic thrombocytopenic purpura". Semin. Hematol. 41 (1): 4–14. PMID 14727254.
- ↑ Bongers T, de Maat M, van Goor M, et. al (2006). "High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability". Stroke. 37 (11): 2672–7. PMID 16990571 PMID 16990571 Check
|pmid=value (help). - ↑ von Willebrand EA (1926). "Hereditär pseudohemofili". Fin Läkaresällsk Handl. 68: 87–112. Reproduced in Von Willebrand EA (1999). "Hereditary pseudohaemophilia". Haemophilia. 5 (3): 223–31, discussion 222. doi:10.1046/j.1365-2516.1999.00302.x. PMID 10444294.
See also
ar:عامل فون ويليبراند de:Von-Willebrand-Faktor sv:Von Willebrands faktor