Vemurafenib
| File:PLX4032 BRAF inhibitor.png | |
| Clinical data | |
|---|---|
| Trade names | Zelboraf |
| Synonyms | PLX4032, RG7204, RO5185426 |
| AHFS/Drugs.com | FDA Professional Drug Information |
| MedlinePlus | a612009 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C23H18ClF2N3O3S |
| Molar mass | 489.92 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
|
WikiDoc Resources for Vemurafenib |
|
Articles |
|---|
|
Most recent articles on Vemurafenib Most cited articles on Vemurafenib |
|
Media |
|
Powerpoint slides on Vemurafenib |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Vemurafenib at Clinical Trials.gov Clinical Trials on Vemurafenib at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Vemurafenib
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Vemurafenib Discussion groups on Vemurafenib Patient Handouts on Vemurafenib Directions to Hospitals Treating Vemurafenib Risk calculators and risk factors for Vemurafenib
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Vemurafenib |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Vemurafenib (INN, marketed as Zelboraf) is a B-Raf enzyme inhibitor developed by Plexxikon (now part of the Daiichi Sankyo group) and Hoffmann–La Roche for the treatment of late-stage melanoma.[1] The name "vemurafenib" comes from V600E mutated BRAF inhibition.
Vemurafenib received FDA approval for the treatment of late-stage melanoma on August 17, 2011,[2] Health Canada approval on February 15, 2012[3] and on February 20, 2012, the European Commission approved vemurafenib as a monotherapy for the treatment of adult patients with BRAF V600 mutation positive unresectable or metastatic melanoma, the most aggressive form of skin cancer.[4]
Mechanism of action
Vemurafenib has been shown to cause programmed cell death in melanoma cell lines.[5] Vemurafenib interrupts the B-Raf/MEK step on the B-Raf/MEK/ERK pathway − if the B-Raf has the common V600E mutation.
Vemurafenib only works in melanoma patients whose cancer has a V600E BRAF mutation (that is, at amino acid position number 600 on the B-Raf protein, the normal valine is replaced by glutamic acid). About 60% of melanomas have this mutation. Melanoma cells without this mutation are not inhibited by vemurafenib; the drug paradoxically stimulates normal BRAF and may promote tumor growth in such cases.[6][7]
In vitro, a melanoma cell line A375 is inhibited by silencing the BRAF gene by short hairpin RNA.[5]
Resistance
Two mechanisms of resistance to vemurafenib (covering 40% of cases) have been discovered:
- The cancer cells begin to overexpress a cell surface protein PDGFRB creating an alternate survival pathway.
- A second oncogene called NRAS mutates, reactivating the normal BRAF survival pathway.[8]
Clinical trials
In a phase I clinical study, vemurafenib was able to reduce numbers of cancer cells in over half of a group of 16 patients with advanced melanoma, and the treated group had a median increased survival time of 6 months over the control group.[9][10][11][12] A second phase I study, in patients with a V600E mutation in B-Raf, ~80% showed partial to complete regression. However the regression only lasted from 2 to 18 months.[13]
Phase I[14] and phase II studies are ongoing,[15] and a phase III trial has been started.[16]
In 2010, it was also in phase I trials for colorectal cancer.[14]
In June 2011, positive results were reported from the phase III BRIM3 BRAF-mutation melanoma study.[17] Further trials are planned including a trial where vemurafenib will be co-administered with GDC-0973, a MEK-inhibitor.[17]
Side effects
At the maximum tolerated dose (MTD) of 960 mg twice a day 31% of patients get skin lesions that may need surgical removal.[1] The BRIM-2 trial investigated 132 patients; the most common adverse events were arthralgia in 58% of patients, skin rash in 52%, and photosensitivity in 52%. In order to better manage side effects some form of dose modification was necessary in 45% of patients. The median daily dose was 1750 mg, which is 91% of the MTD.[18]
References
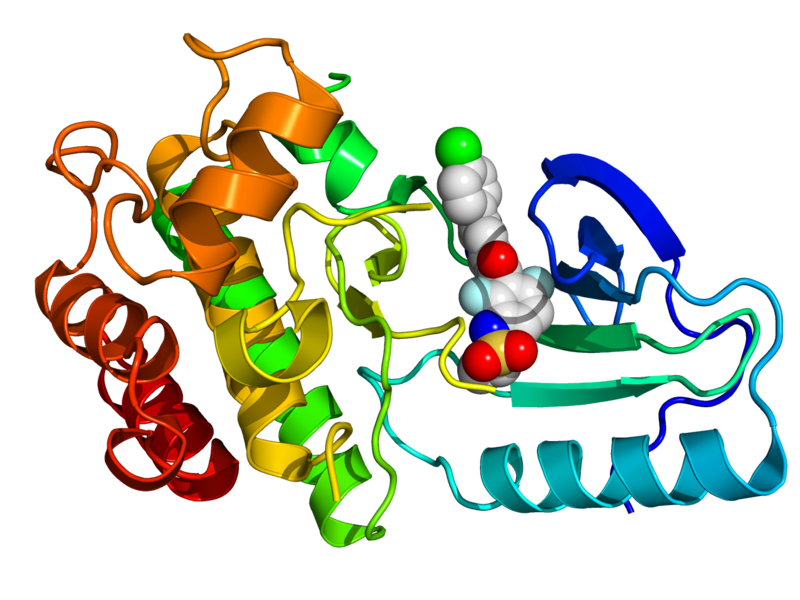
- ↑ 1.0 1.1 1.2 PDB: 3OG7; Bollag G; Hirth P; et al. (2010). "Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma". Nature. 467 (7315): 596–599. doi:10.1038/nature09454. PMC 2948082. PMID 20823850. Unknown parameter
|month=ignored (help); Unknown parameter|author-separator=ignored (help) - ↑ "FDA Approves Zelboraf (Vemurafenib) and Companion Diagnostic for BRAF Mutation-Positive Metastatic Melanoma, a Deadly Form of Skin Cancer" (Press release). Genentech. Retrieved 2011-08-17.
- ↑ Notice of Decision for ZELBORAF
- ↑ First Personalized Cancer Medicine Allows Patients with Deadly Form of Metastatic Melanoma to Live Significantly Longer, Onco'Zine - The International Cancer Network, February 20, 2012
- ↑ 5.0 5.1 Sala E (2008). "BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells". Mol. Cancer Res. 6 (5): 751–9. doi:10.1158/1541-7786.MCR-07-2001. PMID 18458053. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Hatzivassiliou G (2010). "RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth". Nature. 464 (7287): 431–5. doi:10.1038/nature08833. PMID 20130576. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Halaban R (2010). "PLX4032, a Selective BRAF(V600E) Kinase Inhibitor, Activates the ERK Pathway and Enhances Cell Migration and Proliferation of BRAF(WT) Melanoma Cells". Pigment Cell Melanoma Res. 23 (2): 190–200. doi:10.1111/j.1755-148X.2010.00685.x. PMC 2848976. PMID 20149136. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Nazarian R (2010). "Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation". Nature. 468 (7326): 973–977. doi:10.1038/nature09626. PMC 3143360. PMID 21107323. Lay summary – Genetic Engineering & Biotechnology News. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ "Drug hope for advanced melanoma". BBC News. 2009-06-02. Retrieved 2009-06-07.
- ↑ Harmon, Amy (2010-02-21). "A Roller Coaster Chase for a Cure". The New York Times.
- ↑ Garber K (2009). "Melanoma drug vindicates targeted approach". Science. 326 (5960): 1619. doi:10.1126/science.326.5960.1619. PMID 20019269. Unknown parameter
|month=ignored (help) - ↑ Flaherty K. "Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer". 2009 ASCO Annual Meeting Abstract, J Clin Oncol 27:15s, 2009 (suppl; abstr 9000).
- ↑ Flaherty KT (2010). "Inhibition of mutated, activated BRAF in metastatic melanoma". N. Engl. J. Med. 363 (9): 809–19. doi:10.1056/NEJMoa1002011. PMID 20818844. Lay summary – Corante: In the Pipeline. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ 14.0 14.1 "Safety Study of PLX4032 in Patients With Solid Tumors". ClinicalTrials.gov. 2009-10-28. Retrieved 2010-02-23.
- ↑ "A Study of RO5185426 in Previously Treated Patients With Metastatic Melanoma". ClinicalTrials.gov. 2010-02-15. Retrieved 2010-02-23.
- ↑ "Plexxikon Announces First Patient Dosed in Phase 3 Trial of PLX4032 (RG7204) for Metastatic Melanoma" (Press release). Plexxiko. 2010-01-08. Retrieved 2010-03-04.
- ↑ 17.0 17.1 "Plexxikon and Roche Report Positive Data from Phase III BRAF Mutation Melanoma Study". 6 June 2011.
- ↑ "BRIM-2 Upholds Benefits Emerging with Vemurafenib in Melanoma". Oncology & Biotech News. 5 (7). 2011. Unknown parameter
|month=ignored (help)
Template:Extracellular chemotherapeutic agents
- Pages with script errors
- Pages with citations using unsupported parameters
- Pages with broken file links
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drug has EMA link
- Antineoplastic and immunomodulating drugs
- Organochlorides
- Organofluorides
- Sulfonamides
- Tyrosine kinase inhibitors
- Article Feedback 5