Valproic acid: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=peripheral edema,alopecia, rash, increased appetite, weight increased, abdominal pain, constipation, diarrhea, indigestion, loss of appetite, nausea, vomiting, ecchymosis, sthenia, backache, amnesia, ataxia, dizziness, headache, insomnia, somnolence, tremor, amblyopia, blurred vision, diplopia, nystagmus, tinnitus, depression, disturbance in thinking, feeling nervous, mood swings, bronchitis, dyspnea, pharyngitis, respiratory tract infection, rhinitis, fever, influenza | |adverseReactions=peripheral edema,alopecia, rash, increased appetite, weight increased, abdominal pain, constipation, diarrhea, indigestion, loss of appetite, nausea, vomiting, ecchymosis, sthenia, backache, amnesia, ataxia, dizziness, headache, insomnia, somnolence, tremor, amblyopia, blurred vision, diplopia, nystagmus, tinnitus, depression, disturbance in thinking, feeling nervous, mood swings, bronchitis, dyspnea, pharyngitis, respiratory tract infection, rhinitis, fever, influenza | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;"> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">WARNING: LIFE THREATENING ADVERSE REACTIONS</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> ( | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> | ||
Hepatotoxicity | |||
General Population: Hepatic failure resulting in fatalities has occurred in patients receiving valproate. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months [see Warnings and Precautions]. | |||
Children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those on multiple anticonvulsants, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease. When Depakene products are used in this patient group, they should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. The incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups. | |||
Patients with Mitochondrial Disease: There is an increased risk of valproate-induced acute liver failure and resultant deaths in patients with hereditary neurometabolic syndromes caused by DNA mutations of the mitochondrial DNA Polymerase γ (POLG) gene (e.g. Alpers Huttenlocher Syndrome). Depakene is contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and children under two years of age who are clinically suspected of having a mitochondrial disorder [see Contraindications]. In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, Depakene should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with Depakene for the development of acute liver injury with regular clinical assessments and serum liver testing. POLG mutation screening should be performed in accordance with current clinical practice [see Warnings and Precautions]. | |||
Fetal Risk | |||
Valproate can cause major congenital malformations, particularly neural tube defects (e.g., spina bifida). In addition, valproate can cause decreased IQ scores following in utero exposure. | |||
Valproate should only be used to treat pregnant women with epilepsy if other medications have failed to control their symptoms or are otherwise unacceptable. | |||
Valproate should not be administered to a woman of childbearing potential unless the drug is essential to the management of her medical condition. This is especially important when valproate use is considered for a condition not usually associated with permanent injury or death (e.g., migraine). Women should use effective contraception while using valproate [see Warnings and Precautions]. | |||
A Medication Guide describing the risks of valproate is available for patients [see Patient Counseling Information ]. | |||
Pancreatitis | |||
Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with a rapid progression from initial symptoms to death. Cases have been reported shortly after initial use as well as after several years of use. Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, valproate should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated [see Warnings and Precautions]. | |||
|fdaLIADAdult=*Absence seizure, simple and complex | |fdaLIADAdult=*Absence seizure, simple and complex | ||
:* Initial 15 mg/kg/day PO in 2 to 3 divided doses if total daily dose exceeds 250 mg | :* Initial 15 mg/kg/day PO in 2 to 3 divided doses if total daily dose exceeds 250 mg | ||
Revision as of 15:29, 3 June 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LIFE THREATENING ADVERSE REACTIONS
See full prescribing information for complete Boxed Warning.
Condition Name:
Hepatotoxicity General Population: Hepatic failure resulting in fatalities has occurred in patients receiving valproate. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months [see Warnings and Precautions]. Children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those on multiple anticonvulsants, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease. When Depakene products are used in this patient group, they should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. The incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups. Patients with Mitochondrial Disease: There is an increased risk of valproate-induced acute liver failure and resultant deaths in patients with hereditary neurometabolic syndromes caused by DNA mutations of the mitochondrial DNA Polymerase γ (POLG) gene (e.g. Alpers Huttenlocher Syndrome). Depakene is contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and children under two years of age who are clinically suspected of having a mitochondrial disorder [see Contraindications]. In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, Depakene should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with Depakene for the development of acute liver injury with regular clinical assessments and serum liver testing. POLG mutation screening should be performed in accordance with current clinical practice [see Warnings and Precautions]. Fetal Risk Valproate can cause major congenital malformations, particularly neural tube defects (e.g., spina bifida). In addition, valproate can cause decreased IQ scores following in utero exposure. Valproate should only be used to treat pregnant women with epilepsy if other medications have failed to control their symptoms or are otherwise unacceptable. Valproate should not be administered to a woman of childbearing potential unless the drug is essential to the management of her medical condition. This is especially important when valproate use is considered for a condition not usually associated with permanent injury or death (e.g., migraine). Women should use effective contraception while using valproate [see Warnings and Precautions]. A Medication Guide describing the risks of valproate is available for patients [see Patient Counseling Information ]. Pancreatitis Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with a rapid progression from initial symptoms to death. Cases have been reported shortly after initial use as well as after several years of use. Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, valproate should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated [see Warnings and Precautions]. |
Overview
Valproic acid is a anticonvulsant drug that is FDA approved for the {{{indicationType}}} of absence seizure, Simple and complex, complex partial epileptic seizur, manic, bipolar I disorder, migraine; Prophylaxis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include peripheral edema,alopecia, rash, increased appetite, weight increased, abdominal pain, constipation, diarrhea, indigestion, loss of appetite, nausea, vomiting, ecchymosis, sthenia, backache, amnesia, ataxia, dizziness, headache, insomnia, somnolence, tremor, amblyopia, blurred vision, diplopia, nystagmus, tinnitus, depression, disturbance in thinking, feeling nervous, mood swings, bronchitis, dyspnea, pharyngitis, respiratory tract infection, rhinitis, fever, influenza.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Absence seizure, simple and complex
- Initial 15 mg/kg/day PO in 2 to 3 divided doses if total daily dose exceeds 250 mg
- Maintenance increase dosage 5 to 10 mg/kg/day PO at 1-week intervals give in 2 to 3 divided doses if total daily dose exceeds 250 mg max 60 mg/kg/day or less with a therapeutic serum range of 50 to 100 mcg/mL
- Complex partial epileptic seizure
- Initial 10 to 15 mg/kg/day PO in 2 to 3 divided doses if total daily dose exceeds 250 mg, increase dosage 5 to 10 mg/kg/day at 1-week intervals to achieve optimal respons max 60 mg/kg/day or less with a therapeutic serum range of 50 to 100 mcg/mL
- Conversion to monotherapy, 10 to 15 mg/kg/day PO in 2 to 3 divided doses if total daily dose exceeds 250 mg, increase dosage 5 to 10 mg/kg/day at 1-week intervals to achieve optimal clinical response max 60 mg/kg/day or less with a therapeutic serum range of 50 to 100 mcg/mL
- Adjunct may be added to the regimen at an initial dosage of 10 to 15 mg/kg/day PO in 2 to 3 divided doses if total daily dose exceeds 250 mg, increase dosage 5 to 10 mg/kg/day at 1-week intervals to achieve optimal clinical response max 60 mg/kg/day or less.
- Manic bipolar I disorder
- Initial, delayed-release 750 mg PO daily, in divided doses; may increase dose to achieve desired clinical response max 60 mg/kg/day or less
- Migraine Prophylaxis
- Delayed-release 250 mg PO twice daily, max dose 1000 mg/day
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Valproic acid in adult patients.
Non–Guideline-Supported Use
- Alcohol hallucinosis
- Bipolar disorder
- Myelodysplastic syndrome
- Myoclonic seizure
There is limited information about Off-Label Non–Guideline-Supported Use of Valproic acid in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Risk of fatal hepatotoxicity in patients under the age of 2 years
- Absence seizure, Simple and complexfor 2.5 to 13 years
- 10 mg/kg/day for 2 weeks, 15 mg/kg/day for weeks 3 and 4, 20 mg/kg/day for weeks 5 and 6, 30 mg/kg/day for weeks 7 and 8, 40 mg/kg/day for weeks 9 and 10, 50 mg/kg/day for weeks 11 and 12, 60 mg/kg/day for weeks 13 through 16; max dose 60 mg/kg/day or 3000 mg/day, whichever lower, mean dose, 34.9 mg/kg/day
- For 10 years or older, initial, 15 mg/kg/day PO give in 2 to 3 divided doses if dose exceeds 250 mg, maintenance 5 to 10 mg/kg/day PO 1-week intervals until seizures are controlled or side effects preclude further increases in 2 to 3 divided doses if total daily dose exceeds 250 mg max 60 mg/kg/day or less with a therapeutic serum range of 50 to 100 mcg/mL
- Complex partial epileptic seizure 10 years or older
- Monotherapy, initial 10 to 15 mg/kg/day PO give in 2 to 3 divided doses if total daily dose exceeds 250 mg, may increase dosage 5 to 10 mg/kg/day at 1-week intervals to achieve optimal clinical response max 60 mg/kg/day or less
- conversion to monotherapy, 10 to 15 mg/kg/day ORALLY (give in 2 to 3 divided doses if total daily dose exceeds 250 mg), may increase dosage 5 to 10 mg/kg/day at 1-week intervals to achieve optimal clinical response max 60 mg/kg/day or less
- Adjunct, may be added to the regimen at an initial dosage of 10 to 15 mg/kg/day PO in 2 to 3 divided doses if total daily dose exceeds 250 mg, may increase dosage 5 to 10 mg/kg/day at 1-week intervals to achieve optimal clinical response max 60 mg/kg/day.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Valproic acid in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Valproic acid in pediatric patients.
Contraindications
There is limited information regarding Valproic acid Contraindications in the drug label.
Warnings
|
WARNING: LIFE THREATENING ADVERSE REACTIONS
See full prescribing information for complete Boxed Warning.
Condition Name:
Hepatotoxicity General Population: Hepatic failure resulting in fatalities has occurred in patients receiving valproate. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months [see Warnings and Precautions]. Children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those on multiple anticonvulsants, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease. When Depakene products are used in this patient group, they should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. The incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups. Patients with Mitochondrial Disease: There is an increased risk of valproate-induced acute liver failure and resultant deaths in patients with hereditary neurometabolic syndromes caused by DNA mutations of the mitochondrial DNA Polymerase γ (POLG) gene (e.g. Alpers Huttenlocher Syndrome). Depakene is contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and children under two years of age who are clinically suspected of having a mitochondrial disorder [see Contraindications]. In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, Depakene should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with Depakene for the development of acute liver injury with regular clinical assessments and serum liver testing. POLG mutation screening should be performed in accordance with current clinical practice [see Warnings and Precautions]. Fetal Risk Valproate can cause major congenital malformations, particularly neural tube defects (e.g., spina bifida). In addition, valproate can cause decreased IQ scores following in utero exposure. Valproate should only be used to treat pregnant women with epilepsy if other medications have failed to control their symptoms or are otherwise unacceptable. Valproate should not be administered to a woman of childbearing potential unless the drug is essential to the management of her medical condition. This is especially important when valproate use is considered for a condition not usually associated with permanent injury or death (e.g., migraine). Women should use effective contraception while using valproate [see Warnings and Precautions]. A Medication Guide describing the risks of valproate is available for patients [see Patient Counseling Information ]. Pancreatitis Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with a rapid progression from initial symptoms to death. Cases have been reported shortly after initial use as well as after several years of use. Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, valproate should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated [see Warnings and Precautions]. |
There is limited information regarding Valproic acid Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Valproic acid Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Valproic acid Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Valproic acid Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Valproic acid in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Valproic acid in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Valproic acid during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Valproic acid in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Valproic acid in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Valproic acid in geriatric settings.
Gender
There is no FDA guidance on the use of Valproic acid with respect to specific gender populations.
Race
There is no FDA guidance on the use of Valproic acid with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Valproic acid in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Valproic acid in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Valproic acid in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Valproic acid in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Valproic acid Administration in the drug label.
Monitoring
There is limited information regarding Valproic acid Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Valproic acid and IV administrations.
Overdosage
There is limited information regarding Valproic acid overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
There is limited information regarding Valproic acid Mechanism of Action in the drug label.
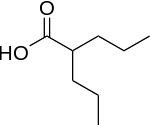
Structure
There is limited information regarding Valproic acid Structure in the drug label.
Pharmacodynamics
There is limited information regarding Valproic acid Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Valproic acid Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Valproic acid Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Valproic acid Clinical Studies in the drug label.
How Supplied
There is limited information regarding Valproic acid How Supplied in the drug label.
Storage
There is limited information regarding Valproic acid Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Valproic acid |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Valproic acid |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Valproic acid Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Valproic acid interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Valproic acid Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Valproic acid Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 "Depakene, Stavzor (valproic acid) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 13 February 2014.