Ulipristal acetate: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 311: | Line 311: | ||
<!--Category--> | <!--Category--> | ||
[[Category:Hormonal Contraception]] | |||
[[Category:Acetate Esters]] | |||
[[Category:Drug]] | [[Category:Drug]] | ||
Revision as of 19:09, 16 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ulipristal acetate is a Contraceptive that is FDA approved for the prevention of pregnancy following unprotected intercourse or a known or suspected contraceptive failure. Common adverse reactions include headache, abdominal pain, nausea, dysmenorrhea, fatigueand dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Ella is a progesterone agonist/antagonist emergency contraceptive indicated for prevention of pregnancy following unprotected intercourse or a known or suspected contraceptive failure. ella is not intended for routine use as a contraceptive.
Dosage
- Instruct patients to take one tablet orally as soon as possible within 120 hours (5 days) after unprotected intercourse or a known or suspected contraceptive failure.
- The tablet can be taken with or without food.
- If vomiting occurs within 3 hours of ella intake, consideration should be given to repeating the dose.
- Ella can be taken at any time during the menstrual cycle.
DOSAGE FORMS AND STRENGTHS
- The ella tablet is supplied as a white to off-white, round, curved tablet containing 30 mg of ulipristal acetate and is marked “ella” on both sides.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Ulipristal acetate in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Ulipristal acetate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There is limited information regarding FDA-Labeled Use of Ulipristal acetate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Ulipristal acetate in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Ulipristal acetate in pediatric patients.
Contraindications
- Ella is contraindicated for use in the case of known or suspected pregnancy. The risks to a fetus when ella is administered to a pregnant woman are unknown. If this drug is inadvertently used during pregnancy, the woman should be apprised of the potential hazard to the fetus.
Warnings
Existing Pregnancy
- Ella is not indicated for termination of an existing pregnancy. Pregnancy should be excluded before prescribing ella. If pregnancy cannot be excluded on the basis of history and/or physical examination, pregnancy testing should be performed. A follow-up physical or pelvic examination is recommended if there is any doubt concerning the general health or pregnancy status of any woman after taking ella.
Ectopic Pregnancy
- A history of ectopic pregnancy is not a contraindication to use of this emergency contraceptive method. Healthcare providers, however, should consider the possibility of ectopic pregnancy in women who become pregnant or complain of lower abdominal pain after taking ella. A follow-up physical or pelvic examination is recommended if there is any doubt concerning the general health or pregnancy status of any woman after taking ella.
Repeated Use
- Ella is for occasional use as an emergency contraceptive. It should not replace a regular method of contraception. Repeated use of ella within the same menstrual cycle is not recommended, as safety and efficacy of repeat use within the same cycle has not been evaluated.
CYP3A4 Inducers
- A CYP3A4 inducer, rifampin, decreases the plasma concentration of ella significantly. Ella should not be administered with CYP3A4 inducers.
Fertility Following Use
- A rapid return of fertility is likely following treatment with ella for emergency contraception; therefore, routine contraception should be continued or initiated as soon as possible following use of ella to ensure ongoing prevention of pregnancy. While there are no data about use of ella with regular hormonal contraceptives, due to its high affinity binding to the progesterone receptor, use of ella may reduce the contraceptive action of regular hormonal contraceptive methods. Therefore, after use of ella, a reliable barrier method of contraception should be used with subsequent acts of intercourse that occur in that same menstrual cycle.
Effect on Menstrual Cycle
- After ella intake, menses sometimes occur earlier or later than expected by a few days. In clinical trials, cycle length was increased by a mean of 2.5 days but returned to normal in the subsequent cycle. Seven percent of subjects reported menses occurring more than 7 days earlier than expected, and 19% reported a delay of more than 7 days. If there is a delay in the onset of expected menses beyond 1 week, rule out pregnancy.
- Nine percent of women studied reported intermenstrual bleeding after use of ella.
Sexually Transmitted Infections/HIV
- Ella does not protect against HIV infection (AIDS) or other sexually transmitted infections (STIs).
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
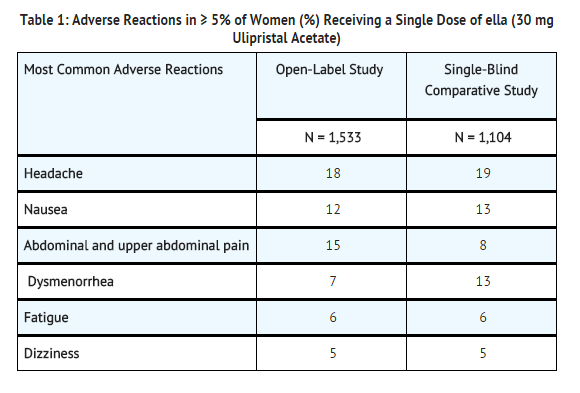
- Ella was studied in an open-label multicenter trial (Open-Label Study) and in a comparative, randomized, single-blind, multicenter trial (Single-Blind Comparative Study). In these studies, a total of 2,637 (1,533 + 1,104) women in the 30 mg ulipristal acetate groups were included in the safety analysis. The mean age of women who received ulipristal acetate was 24.5 years and the mean body mass index (BMI) was 25.3. The racial demographics of those enrolled were 67% Caucasian, 20% Black or African American, 2% Asian, and 12% other.
- The most common adverse reactions (≥ 10%) in the clinical trials for women receiving ella were headache (18% overall) and nausea (12% overall) and abdominal and upper abdominal pain (12% overall). Table 1 lists those adverse reactions that were reported in ≥ 5% of subjects in the clinical studies.
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of ella:
- Skin and Subcutaneous Tissue Disorders: Acne
- Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Drug Interactions
- Several in vivo drug interaction studies have shown that ella is predominantly metabolized by CYP3A4.
Changes in Emergency Contraceptive Effectiveness Associated with Co-Administration of Other Products
- Drugs or herbal products that induce CYP3A4 decrease the plasma concentrations of ella, and may decrease its effectiveness. Avoid co-administration of ella and drugs or herbal products such as:
- Barbiturates
- Bosentan
- Carbamazepine
- Felbamate
- Griseofulvin
- Oxcarbazepine
- Phenytoin
- Rifampin
- St. John’s Wort
- Topiramate
Increase in Plasma Concentrations of ella Associated with Co-Administered Drugs
- CYP3A4 inhibitors such as itraconazole or ketoconazole increase plasma concentrations of ella.
Effects of ella on Co-Administered Drugs
- In vitro studies demonstrated that ella does not induce or inhibit the activity of cytochrome P450 enzymes.
- P-glycoprotein (P-gp) transporters: In vitro data indicate that ulipristal may be an inhibitor of P-gp at clinically relevant concentrations. Thus, co-administration of ulipristal acetate and P-gp substrates (e.g., dabigatran etexilate, digoxin) may increase the concentration of P-gp substrates. In vivo data suggest that ulipristal acetate 10 mg does not affect P-gp transporters. However, there was no in vivo drug interaction study between ulipristal acetate 30 mg and P-gp transporters
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category X.
- Use of ella is contraindicated during an existing or suspected pregnancy.
- There are no adequate and well controlled studies in pregnant women.
- Ulipristal acetate was administered repeatedly to pregnant rats and rabbits during the period of organogenesis. Embryofetal loss was noted in all pregnant rats and in half of the pregnant rabbits following 12 and 13 days of dosing, at daily drug exposures 1/3 and 1/2 the human exposure, respectively, based on body surface area (mg/m2). There were no malformations of the surviving fetuses in these studies. Adverse effects were not observed in the offspring of pregnant rats administered ulipristal acetate during the period of organogenesis through lactation at drug exposures 1/24 the human exposure based on AUC. Administration of ulipristal acetate to pregnant monkeys for 4 days during the first trimester caused pregnancy termination in 2/5 animals at daily drug exposures 3 times the human exposure based on body surface area.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ulipristal acetate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ulipristal acetate during labor and delivery.
Nursing Mothers
- The breast milk of 12 lactating women following administration of ella was collected in 24-hour increments to measure the concentrations of ulipristal acetate and monodemethyl-ulipristal acetate in breast milk. The mean daily concentrations of ulipristal acetate in breast milk were 22.7 ng/mL [0-24 hours], 2.96 ng/mL [24-48 hours], 1.56 ng/mL [48-72 hours], 1.04 ng/mL [72-96 hours], and 0.69 ng/mL [96-120 hours]. The mean daily concentrations of monodemethyl-ulipristal acetate in breast milk were 4.49 ng/mL [0-24 hours], 0.62 ng/mL [24-48 hours], 0.28 ng/mL [48-72 hours], 0.17 ng/mL [72-96 hours], and 0.10 ng/mL [96-120 hours].
- The effect of this exposure on newborns/infants has not been studied; therefore, risk to the breast-fed child cannot be excluded. Therefore, use of ella by breastfeeding women is not recommended.
Pediatric Use
- Safety and efficacy of ella have been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents less than 18 years and for users 18 years and older. Use of ella before menarche is not indicated.
Geriatic Use
- This product is not intended for use in postmenopausal women.
Gender
There is no FDA guidance on the use of Ulipristal acetate with respect to specific gender populations.
Race
- While no formal studies have evaluated the effect of race, a cross-study comparison of two pharmacokinetic studies indicated that exposure in South Asians may exceed that in Caucasians and African Americans. However, no difference in efficacy and safety was observed for women of different races in clinical studies.
Renal Impairment
- No studies have been conducted to evaluate the effect of renal disease on the disposition of ella.
Hepatic Impairment
- No studies have been conducted to evaluate the effect of hepatic disease on the disposition of ella.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ulipristal acetate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ulipristal acetate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Ulipristal acetate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Ulipristal acetate in the drug label.
Overdosage
- Experience with ulipristal acetate overdose is limited. In a clinical study, single doses equivalent to up to 4 times ella were administered to a limited number of subjects without any adverse reactions.
Pharmacology
Mechanism of Action
- When taken immediately before ovulation is to occur, ella postpones follicular rupture. The likely primary mechanism of action of ulipristal acetate for emergency contraception is therefore inhibition or delay of ovulation; however, alterations to the endometrium that may affect implantation may also contribute to efficacy.
Structure
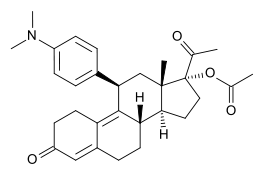
- The ella (ulipristal acetate) tablet for oral use contains 30 mg of a single active steroid ingredient, ulipristal acetate [17α-acetoxy-11β-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione], a synthetic progesterone agonist/antagonist. The inactive ingredients are lactose monohydrate, povidone K-30, croscarmellose sodium and magnesium stearate.
- Ulipristal acetate is a white to yellow crystalline powder which has a molecular weight of 475.6. The structural formula is:

Pharmacodynamics
- Ulipristal acetate is a selective progesterone receptor modulator with antagonistic and partial agonistic effects (a progesterone agonist/antagonist) at the progesterone receptor. It binds the human progesterone receptor and prevents progesterone from occupying its receptor.
- The pharmacodynamics of ulipristal acetate depends on the timing of administration in the menstrual cycle. Administration in the mid-follicular phase causes inhibition of folliculogenesis and reduction of estradiol concentration. Administration at the time of the luteinizing hormone peak delays follicular rupture by 5 to 9 days. Dosing in the early luteal phase does not significantly delay endometrial maturation but decreases endometrial thickness by 0.6 ± 2.2 mm (mean ± SD).
Pharmacokinetics
Absorption
- Following a single dose administration of ella in 20 women under fasting conditions, maximum plasma concentrations of ulipristal acetate and the active metabolite, monodemethyl-ulipristal acetate, were 176 and 69 ng/ml and were reached at 0.9 and 1 hour, respectively.


- Effect of food: Administration of ella together with a high-fat breakfast resulted in approximately 40-45% lower mean Cmax, a delayed tmax (from a median of 0.75 hours to 3 hours) and 20-25% higher mean AUC0-∞ of ulipristal acetate and monodemethyl-ulipristal acetate compared with administration in the fasting state. These differences are not expected to impair the efficacy or safety of ella to a clinically significant extent; therefore, ella can be taken with or without food.
Distribution
- Ulipristal acetate is highly bound (> 94%) to plasma proteins, including high density lipoprotein, alpha-l-acid glycoprotein, and albumin.
Metabolism
- Ulipristal acetate is metabolized to mono-demethylated and di-demethylated metabolites. In vitro data indicate that this is predominantly mediated by CYP3A4. The mono-demethylated metabolite is pharmacologically active.
Excretion
- The terminal half-life of ulipristal acetate in plasma following a single 30 mg dose is estimated to 32.4 ± 6.3 hours.
Drug interactions
- CYP3A4 inducers: When a single 30 mg dose of ulipristal acetate was administered following administration of the strong CYP3A4 inducer, rifampin 600 mg once daily for 9 days, Cmax and AUC of ulipristal acetate decreased by 90% and 93% respectively. The Cmax and AUC of monodemethyl-ulipristal acetate decreased by 84% and 90% respectively.
- CYP3A4 inhibitors: When a single 10 mg dose of ulipristal acetate was administered following administration of the strong CYP3A4 inhibitor, ketoconazole 400 mg once daily for 7 days, Cmax and AUC of ulipristal acetate increased by 2- and 5.9- fold, respectively. While the AUC of monodemethyl-ulipristal acetate increased by 2.4-fold, Cmax of monodemethyl-ulipristal acetate decreased by 47%. There was no in vivo drug-drug interaction study between ulipristal acetate 30 mg and CYP3A4 inhibitors [see Drug Interactions (7.1)].
- P-glycoprotein (P-gp) transporters: When a single 60 mg dose of fexofenadine, a substrate of P-gp glycoprotein, was administered 1.5 hours after the administration of a single 10 mg dose of ulipristal acetate, there was no increase in Cmax or AUC of fexofenadine.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity: Carcinogenicity studies with ulipristal acetate have not been conducted.
- Genotoxicity: Ulipristal acetate was not genotoxic in the Ames assay, in vitro mammalian assays utilizing mouse lymphoma cells and human peripheral blood lymphocytes, and in an in vivo micronucleus assay in mice.
- Impairment of Fertility: Single oral doses of ulipristal acetate prevented ovulation in 50% of rats at 2 times the human exposure based on body surface area (mg/m2). Single doses of ulipristal acetate given on post-coital days 4 or 5 prevented pregnancy in 80 - 100% of rats and in 50% of rabbits when given on post-coital days 5 or 6 at drug exposures 4 and 12 times the human exposure based on body surface area. Lower doses administered for 4 days to rats and rabbits were also effective at preventing ovulation and pregnancy.
Clinical Studies
- Two multicenter clinical studies evaluated the efficacy and safety of ella. An open-label study provided the primary data to support the efficacy and safety of ulipristal acetate for emergency contraception when taken 48 to 120 hours after unprotected intercourse. A single-blind comparative study provided the primary data to support the efficacy and safety of ulipristal acetate for emergency contraception when taken 0 to 72 hours after unprotected intercourse and provided supportive data for ulipristal acetate for emergency contraception when taken > 72 to 120 hours after unprotected intercourse. Women in both studies were required to have a negative pregnancy test prior to receiving emergency contraception. The primary efficacy analyses were performed on subjects less than 36 years of age who had a known pregnancy status after taking study medication.

Open-Label Study
- This study was a multicenter open-label trial conducted at 40 family planning clinics in the United States. Healthy women with a mean age of 24 years who requested emergency contraception 48 to 120 hours after unprotected intercourse received a dose of 30 mg ulipristal acetate (ella). The median BMI for the study subjects was 25.3 and ranged from 16.1 to 61.3 kg/m2.
- Twenty-seven pregnancies occurred in 1,242 women aged 18 to 35 years evaluated for efficacy. The number of pregnancies expected without emergency contraception was calculated based on the timing of intercourse with regard to each woman’s menstrual cycle. ella statistically significantly reduced the pregnancy rate, from an expected rate of 5.5% to an observed rate of 2.2%, when taken 48 to 120 hours after unprotected intercourse.
Single-Blind Comparative Study
- This study was a multicenter, single-blind, randomized comparison of the efficacy and safety of 30 mg ulipristal acetate (ella) to levonorgestrel (another form of emergency contraception). Subjects were enrolled at 35 sites in the U.S., the United Kingdom and Ireland, with the majority (66%) having been enrolled in the U.S. Healthy women with a mean age of 25 years who requested emergency contraception within 120 hours of unprotected intercourse were enrolled and randomly allocated to receive ella or levonorgestrel 1.5 mg. The median BMI for the study subjects was 25.3 and ranged from 14.9 to 70.0 kg/m2.
- In the ella group, 16 pregnancies occurred in 844 women aged 16 to 35 years when emergency contraception was taken 0 to 72 hours after unprotected intercourse. The number of pregnancies expected without emergency contraception was calculated based on the timing of intercourse with regard to each woman’s menstrual cycle; ella statistically significantly reduced the pregnancy rate, from an expected 5.6% to an observed 1.9%, when taken within 72 hours after unprotected intercourse. There were no pregnancies observed in the women who were administered ella more than 72 hours after unprotected intercourse (10% of women who received ella).
Pooled Analysis
- Data from the two studies were pooled to provide a total efficacy population of women treated with ulipristal acetate up to 120 hours after UPI. Time Trend analysis for the five 24-hour intervals from 0 to 120 hours between unprotected intercourse and treatment was conducted. There were no significant differences in the observed pregnancy rates across the five time intervals.
- Subgroup analysis of the pooled data by BMI showed that for women with BMI > 30 kg/m2 (16% of all subjects), the observed pregnancy rate was 3.1% (95% CI: 1.7, 5.7), which was not significantly reduced compared to the expected pregnancy rate of 4.5% in the absence of emergency contraception taken within 120 hours after unprotected intercourse. In the comparative study, a similar effect was seen for the comparator emergency contraception drug, levonorgestrel 1.5 mg. For levonorgestrel, when used by women with BMI > 30 kg/m2, the observed pregnancy rate was 7.4% (95% CI: 3.9, 13.4), compared to the expected pregnancy rate of 4.4% in the absence of emergency contraception taken within 72 hours after unprotected intercourse.
How Supplied
- Ella (ulipristal acetate) tablet, 30 mg is supplied in a PVC-PE-PVDC-aluminum blister. The tablet is a white to off-white, round, curved tablet marked with "ella" on both sides.
- NDC 50102-911-01 (1 tablet unit of use package)
Storage
- Store at 20-25°C (68-77°F).
Images
Drug Images
{{#ask: Page Name::Ulipristal acetate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Ulipristal acetate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients
- Instruct patients to take ella as soon as possible and not more than 120 hours after unprotected intercourse or a known or suspected contraceptive failure.
- Advise patients that they should not take ella if they know or suspect they are pregnant and that ella is not indicated for termination of an existing pregnancy.
- Advise patients to contact their healthcare provider immediately in case of vomiting within 3 hours of taking the tablet, to discuss whether to take another tablet.
- Advise patients to seek medical attention if they experience severe lower abdominal pain 3 to 5 weeks after taking ella, in order to be evaluated for an ectopic pregnancy.
- Advise patients to contact their healthcare provider and consider the possibility of pregnancy if their period is delayed after taking ella by more than 1 week beyond the date it was expected.
- Advise patients not to use ella as routine contraception, or to use it repeatedly in the same menstrual cycle.
- Advise patients that ella may reduce the contraceptive action of regular hormonal contraceptive methods and to use a reliable barrier method of contraception after using ella, for any subsequent acts of intercourse that occur in that same menstrual cycle.
- Advise patients not to use ella if they are taking a CYP3A4 inducer.
- Inform patients that ella does not protect against HIV infection (AIDS) and other sexually transmitted diseases/infections.
- Advise patients that they should not use ella if they are breastfeeding because ella enters the breast milk.
Precautions with Alcohol
- Alcohol-Ulipristal acetate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ELLA®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "ELLA- ulipristal acetate tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Ulipristal acetate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Ulipristal acetate |Label Name=Ulipristal acetate11.png
}}
{{#subobject:
|Label Page=Ulipristal acetate |Label Name=Ulipristal acetate11.png
}}