Tuberous sclerosis pathophysiology
|
Tuberous sclerosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberous sclerosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Tuberous sclerosis pathophysiology |
|
Risk calculators and risk factors for Tuberous sclerosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Hamartin and tuberin, which are encoded by TSC1 and TSC2 genes respectively, function as a complex which is involved in the control of cell growth and cell division. (The complex appears to be a Rheb GTPase which suppresses mTOR signaling, part of the growth factor (insulin) signaling pathway.) Thus, mutations at the TSC1 and TSC2 loci result in a loss of control of cell growth and cell division, and therefore a predisposition to forming tumors.
Pathophysiology
Genetics
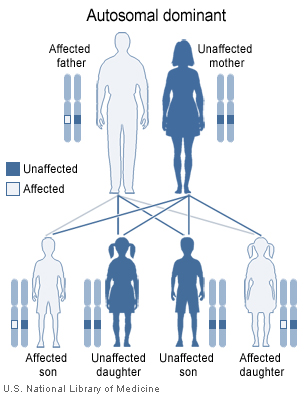
Tuberous sclerosis is a genetic disorder with an autosomal dominant pattern of inheritance, and penetrance is 100%.[1] Two thirds of TSC cases result from sporadic genetic mutations, not inheritance, but their offspring may inherit it from them. Current genetic tests have difficulty locating the mutation in approximately 20% of individuals diagnosed with the disease. So far it has been mapped to two genetic loci, TSC1 and TSC2.
TSC1 encodes for the protein hamartin, is located on chromosome 9 q34 and was discovered in 1997.[2] TSC2 encodes for the protein tuberin, is located on chromosome 16 p13.3 and was discovered in 1993.[3] TSC2 is contiguous with PKD1, the gene involved in one form of polycystic kidney disease (PKD). Gross deletions affecting both genes may account for the 2% of individuals with TSC who also develop PKD in childhood.[4] TSC2 has been associated with a more severe form of TSC.[5] However, the difference is subtle and cannot be used to identify the mutation clinically. Estimates of the proportion of TSC caused by TSC2 range from 55% to 80-90%.[6]
TSC1 and TSC2 are both tumor suppressor genes that function according to Knudson's "two hit" hypothesis. That is, a second random mutation must occur before a tumor can develop. This explains why, despite its 100 percent penetrance, TSC has wide expressivity.
|
| ||||||||||||||||||||||||||||||||||||||||
Pathology
Skin
Some form of dermatological sign will be present in 96% of individuals with TSC. The most common skin abnormalities include:
- Facial angiofibromas: A rash of reddish spots or bumps, which appear on the nose and cheeks in a butterfly distribution. They consist of blood vessels and fibrous tissue. It starts to appear during childhood.
- Ungual or subungual fibromas: Small fleshy tumors that grow around and under the toenails or fingernailsThese are very rare in childhood but common by middle age.
- Hypomelanic macules ("ash leaf spots"): White or lighter patches of skin that may appear anywhere on the body and are caused by a lack of melanin. These are usually the only visible sign of TSC at birth.
- Forehead plaques: Raised, discolored areas on the forehead.
- Shagreen patches: Areas of thick leathery skin that are dimpled like an orange peel, usually found on the lower back or nape of the neck.
- Other skin features are not unique to individuals with TSC, including molluscum fibrosum or skin tags, which typically occur across the back of the neck and shoulders, cafe-au-lait spots or flat brown marks, and poliosis, a tuft or patch of white hair on the scalp or eyelids.
Eyes
Retinal lesions, called astrocytic hamartomas, which appear as a greyish or yellowish-white lesion in the back of the globe on the ophthalmic examination. Astrocytic hamartomas can calcify, and is in the differential diagnosis of a calcified globe mass on a CT scan.
Non-retinal lesions associated with TSC include:
- Coloboma
- Angiofibromas of the eyelids
- Papilledema (related to hydrocephalus)
Heart
Rhabdomyomas are benign tumors of striated muscle. Most commonly they arise from the ventricular myocardium. Other sites of involvement include atria and pericardium. Their range from 1 mm to 10 cm in size. Problems due to rhabdomyomas include obstruction, arrhythmia and a murmur.
Lung
Patients with TSC can develop progressive replacement of the lung parenchyma with multiple cysts. This process is identical to another disease called lymphangioleiomyomatosis (LAM). Recent genetic analysis has shown that the proliferative bronchiolar smooth muscle in tuberous sclerosis-related LAM is monoclonal metastasis from a coexisting renal angiomyolipoma. There have been cases of TSC-related LAM recurring following lung transplant. [7]
Kidneys
Between 60 and 80% of TSC patients have benign tumors (hamartomas) of the kidneys called angiomyolipomas (AML). These tumors are composed of vascular tissue (angio–), smooth muscle (–myo–), and fat(–lipoma). Although benign, an AML larger than 4 cm is at risk for a potentially catastrophic hemorrhage either spontaneously or with minimal trauma. AMLs are found in about 1 in 300 people without TSC. However those are usually solitary, whereas in TSC they are commonly multiple and bilateral.
Neurologic
Classic intracranial manifestations of tuberous sclerosis include subependymal nodules and cortical/subcortical tubers.[8]
Subependymal nodules are composed of abnormal, swollen glial cells and bizarre multinucleated cells which are indeterminate for glial or neuronal origin. There is no interposed neural tissue. These nodules have a tendency to calcify as the patient ages. A nodule that markedly enhances and enlarges over time should be considered suspicious for transformation into a subependymal giant cell astrocytoma (SEGA). A SEGA typically develops in the region of the foramen of Monroe, in which case it is at risk of developing an obstructive hydrocephalus.[9]
A variable degree of ventricular enlargement, either obstructive (e.g. by a subependymal nodule in the region of the foramen of Monroe) or idiopathic in nature.
References
- ↑ Northrup H, Au K (5 December 2005). "Tuberous Sclerosis Complex". GeneReviews. Retrieved 2007-09-02. Check date values in:
|date=(help) - ↑ van Slegtenhorst M, de Hoogt R, Hermans C; et al. (1997). "Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34". Science. 277 (5327): 805–8. PMID 9242607.
- ↑ European Chromosome 16 Tuberous Sclerosis Consortium (1993). "Identification and characterization of the tuberous sclerosis gene on chromosome 16". Cell. 75 (7): 1305–15. PMID 8269512.
- ↑ Brook-Carter PT; et al. (1994). "Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease--a contiguous gene syndrome". Nature Genetics. 8 (4): 328–32. PMID 7894481.
- ↑ Dabora SL; et al. (2001). "Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs". American Journal of Human Genetics. 68 (1): 64–80. PMID 11112665.
- ↑ Rendtorff ND; et al. (2005). "Analysis of 65 tuberous sclerosis complex (TSC) patients by TSC2 DGGE, TSC1/TSC2 MLPA, and TSC1 long-range PCR sequencing, and report of 28 novel mutations". Human Mutation. 26 (4): 374–83. PMID 16114042.
- ↑ Henske EP. (2003). "Metastasis of benign tumor cells in tuberous sclerosis complex". Genes, Chromosomes & Cancer. 38 (4): 376–381. PMID 14566858.
- ↑ Ridler K; et al. (2004). "Standardized whole brain mapping of tubers and subependymal nodules in tuberous sclerosis complex". Journal of Child Neurology. 19 (9): 658–665. PMID 15563011.
- ↑ Grajkowska W, Kotulska K, Jurkiewicz E, Matyja E (2010). "Brain lesions in tuberous sclerosis complex. Review". Folia Neuropathol. 48 (3): 139–49. PMID 20924998.