Trioxsalen: Difference between revisions
No edit summary |
m (Protected "Trioxsalen": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
(No difference)
| |
Latest revision as of 17:21, 20 August 2015
| |
| Names | |
|---|---|
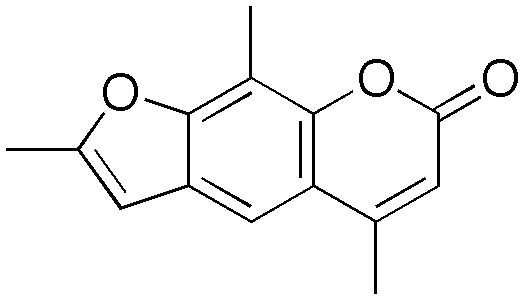
| IUPAC name
2,5,9-trimethyl-7H-furo[3,2-g]chromen-7-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.24328 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Trioxsalen |
|
Articles |
|---|
|
Most recent articles on Trioxsalen |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Trioxsalen at Clinical Trials.gov Clinical Trials on Trioxsalen at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Trioxsalen
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Trioxsalen Discussion groups on Trioxsalen Patient Handouts on Trioxsalen Directions to Hospitals Treating Trioxsalen Risk calculators and risk factors for Trioxsalen
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Trioxsalen |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Trioxsalen (trimethylpsoralen, Trioxysalen or Trisoralen) is a furanocoumarin and a psoralen derivative. It is obtained from several plants, mainly Psoralea corylifolia. Like other psoralens it causes photosensitization of the skin. It is administered either topically or orally in conjunction with UV-A (the least damaging form of ultraviolet light) for phototherapy treatment of vitiligo[1] and hand eczema.[2] After photoactivation it creates interstrand cross-links in DNA, which can cause programmed cell death unless repaired by cellular mechanisms. In research it can be conjugated to dyes for confocal microscopy and used to visualize sites of DNA damage.[3] The compound is also being explored for development of antisense oligonucleotides that can be cross-linked specifically to a mutant mRNA sequence without affecting normal transcripts differing at even a single base pair.[4]
References
- ↑ "Trioxsalen - Compound Summary". PubChem.
- ↑ Van Coevorden, AM; Kamphof, WG; Van Sonderen, E; Bruynzeel, DP; Coenraads, PJ (2004). "Comparison of oral psoralen-UV-A with a portable tanning unit at home vs hospital-administered bath psoralen-UV-A in patients with chronic hand eczema: an open-label randomized controlled trial of efficacy". Archives of dermatology. 140 (12): 1463–6. doi:10.1001/archderm.140.12.1463. PMID 15611423.
- ↑ Thazhathveetil, AK; Liu, ST; Indig, FE; Seidman, MM (2007). "Psoralen conjugates for visualization of genomic interstrand cross-links localized by laser photoactivation". Bioconjugate chemistry. 18 (2): 431–7. doi:10.1021/bc060309t. PMID 17373769.
- ↑ Higuchi, M; Yamayoshi, A; Kobori, A; Yamaoka, T; Murakami, A (2005). "Synthesis and properties of photo-reactive antisense oligonucleotides containing 2'-O-psoralen-conjugated adenosine". Nucleic acids symposium series (2004). 49 (49): 331–2. doi:10.1093/nass/49.1.331. PMID 17150768.