Trientine: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
m (Protected "Trientine": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (9 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{KS}} | |authorTag={{KS}} | ||
|genericName= | |genericName=trientine hydrochloride | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass= | |drugClass=heavy metal chelator | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication= | |indication=[[Wilson's disease]] | ||
|adverseReactions=[[iron deficiency]], [[systemic lupus erythematosus]],[[dystonia]], [[muscular spasm]], [[myasthenia gravis]]. | |||
|blackBoxWarningTitle=Warning Title | |blackBoxWarningTitle=Warning Title | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
| Line 23: | Line 24: | ||
* It is important that SYPRINE be given on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed. | * It is important that SYPRINE be given on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed. | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Trientine in adult patients. | ||
|offLabelAdultNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|fdaLIADPed===Indications== | |||
* SYPRINE is indicated in the treatment of patients with [[Wilson's disease]] who are intolerant of penicillamine. Clinical experience with SYPRINE is limited and alternate dosing regimens have not been well-characterized; all endpoints in determining an individual patient's dose have not been well defined. SYPRINE and penicillamine cannot be considered interchangeable. SYPRINE should be used when continued treatment with penicillamine is no longer possible because of intolerable or life endangering side effects. | |||
* | * Unlike penicillamine, SYPRINE is not recommended in cystinuria or rheumatoid arthritis. The absence of a sulfhydryl moiety renders it incapable of binding cystine and, therefore, it is of no use in cystinuria. In 15 patients with rheumatoid arthritis, SYPRINE was reported not to be effective in improving any clinical or biochemical parameter after 12 weeks of treatment. | ||
* SYPRINE is not indicated for treatment of [[biliary cirrhosis]]. | |||
== | ==Dosage== | ||
* | * Systemic evaluation of dose and/or interval between dose has not been done. However, on limited clinical experience, the recommended initial dose of SYPRINE is 500-750 mg/day for pediatric patients and 750-1250 mg/day for adults given in divided doses two, three or four times daily. This may be increased to a maximum of 2000 mg/day for adults or 1500 mg/day for pediatric patients age 12 or under. | ||
* The daily dose of SYPRINE should be increased only when the clinical response is not adequate or the concentration of free serum copper is persistently above 20 mcg/dL. Optimal long-term maintenance dosage should be determined at 6-12 month intervals. | |||
* | * It is important that SYPRINE be given on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed. | ||
|offLabelPedGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
* | |offLabelPedNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedNoGuideSupport= | |||
* | |||
|contraindications=* [[Hypersensitivity]] to this product. | |contraindications=* [[Hypersensitivity]] to this product. | ||
|warnings=* Patient experience with trientine hydrochloride is limited. Patients receiving SYPRINE should remain under regular medical supervision throughout the period of drug administration. Patients (especially women) should be closely monitored for evidence of [[iron deficiency anemia]]. | |warnings=* Patient experience with trientine hydrochloride is limited. Patients receiving SYPRINE should remain under regular medical supervision throughout the period of drug administration. Patients (especially women) should be closely monitored for evidence of [[iron deficiency anemia]]. | ||
| Line 81: | Line 48: | ||
* SYPRINE is not indicated for treatment of [[biliary cirrhosis]], but in one study of 4 patients treated with trientine hydrochloride for [[primary biliary cirrhosis]], the following adverse reactions were reported: [[heartburn]]; [[epigastric pain]] and tenderness; thickening, fissuring and flaking of the skin; [[hypochromic microcytic anemia]]; acute [[gastritis]]; [[aphthoid ulcers]]; [[abdominal pain]]; [[melena]]; [[anorexia]]; [[malaise]]; [[cramps]]; [[muscle pain]]; [[weakness]]; [[rhabdomyolysis]]. A causal relationship of these reactions to drug therapy could not be rejected or established. | * SYPRINE is not indicated for treatment of [[biliary cirrhosis]], but in one study of 4 patients treated with trientine hydrochloride for [[primary biliary cirrhosis]], the following adverse reactions were reported: [[heartburn]]; [[epigastric pain]] and tenderness; thickening, fissuring and flaking of the skin; [[hypochromic microcytic anemia]]; acute [[gastritis]]; [[aphthoid ulcers]]; [[abdominal pain]]; [[melena]]; [[anorexia]]; [[malaise]]; [[cramps]]; [[muscle pain]]; [[weakness]]; [[rhabdomyolysis]]. A causal relationship of these reactions to drug therapy could not be rejected or established. | ||
|postmarketing=* There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
|drugInteractions=* In general, mineral supplements should not be given since they may block the absorption of SYPRINE. However, iron deficiency may develop, especially in children and menstruating or pregnant women, or as a result of the low copper diet recommended for Wilson's disease. If necessary, iron may be given in short courses, but since iron and SYPRINE each inhibit absorption of the other, two hours should elapse between administration of SYPRINE and iron. | |drugInteractions=* In general, mineral supplements should not be given since they may block the absorption of SYPRINE. However, iron deficiency may develop, especially in children and menstruating or pregnant women, or as a result of the low copper diet recommended for Wilson's disease. If necessary, iron may be given in short courses, but since iron and SYPRINE each inhibit absorption of the other, two hours should elapse between administration of SYPRINE and iron. | ||
* It is important that SYPRINE be taken on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. This permits maximum absorption and reduces the likelihood of inactivation of the drug by metal binding in the gastrointestinal tract. | * It is important that SYPRINE be taken on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. This permits maximum absorption and reduces the likelihood of inactivation of the drug by metal binding in the gastrointestinal tract. | ||
|useInPregnancyFDA='''Pregnancy Category C''' | |useInPregnancyFDA='''Pregnancy Category C''' | ||
* Trientine hydrochloride was teratogenic in rats at doses similar to the human dose. The frequencies of both resorptions and fetal abnormalities, including hemorrhage and edema, increased while fetal copper levels decreased when trientine hydrochloride was given in the maternal diets of rats. There are no adequate and well-controlled studies in pregnant women. SYPRINE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | * Trientine hydrochloride was teratogenic in rats at doses similar to the human dose. The frequencies of both resorptions and fetal abnormalities, including hemorrhage and edema, increased while fetal copper levels decreased when trientine hydrochloride was given in the maternal diets of rats. There are no adequate and well-controlled studies in pregnant women. SYPRINE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | ||
|useInPregnancyAUS=* There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=* There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SYPRINE is administered to a nursing mother. | |useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SYPRINE is administered to a nursing mother. | ||
|useInPed=* Controlled studies of the safety and effectiveness of SYPRINE in pediatric patients have not been conducted. It has been used clinically in pediatric patients as young as 6 years with no reported adverse experiences. | |useInPed=* Controlled studies of the safety and effectiveness of SYPRINE in pediatric patients have not been conducted. It has been used clinically in pediatric patients as young as 6 years with no reported adverse experiences. | ||
|useInGeri=* Clinical studies of SYPRINE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience is insufficient to determine differences in responses between the elderly and younger patients. In general, dose selection should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. | |useInGeri=* Clinical studies of SYPRINE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience is insufficient to determine differences in responses between the elderly and younger patients. In general, dose selection should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. | ||
|useInGender=* There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=* There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=* There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=* There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=* There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=* There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
|othersTitle=Others | |othersTitle=Others | ||
|administration=* Oral | |||
|administration= | |||
* | * Patients should be directed to take SYPRINE on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed. Because of the potential for contact dermatitis, any site of exposure to the capsule contents should be washed with water promptly. For the first month of treatment, the patient should have his temperature taken nightly, and he should be asked to report any symptom such as fever or skin eruption. | ||
|monitoring=* There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat=* There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
* | |||
= | |||
* | |||
|overdose====Acute Overdose=== | |overdose====Acute Overdose=== | ||
* There is a report of an adult woman who ingested 30 grams of trientine hydrochloride without apparent ill effects. No other data on overdosage are available. | |||
|drugBox=[[File:Triethylinetramine wikipedia.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|mechAction=* There is limited information regarding <i>Mechanism Of Action</i> of {{PAGENAME}} in the drug label. | |||
|structure=* Trientine hydrochloride is N,N'-bis (2-aminoethyl)-1,2-ethanediamine dihydrochloride. It is a white to pale yellow crystalline hygroscopic powder. It is freely soluble in water, soluble in methanol, slightly soluble in ethanol, and insoluble in chloroform and ether. | |||
|drugBox= | |||
| | |||
| | |||
| | |||
| | |||
The empirical formula is C6H18N4•2HCl with a molecular weight of 219.2. The structural formula is: | |||
NH2(CH2)2NH(CH2)2NH(CH2)2NH2•2HCl | |||
Trientine hydrochloride is a chelating compound for removal of excess copper from the body. SYPRINE1 (Trientine Hydrochloride) is available as 250 mg capsules for oral administration. Capsules SYPRINE contain gelatin, iron oxides, stearic acid, and titanium dioxide as inactive ingredients. | |||
| | |PD=* There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
|PK=* Data on the pharmacokinetics of trientine hydrochloride are not available. Dosage adjustment recommendations are based upon clinical use of the drug. | |||
< | |||
}} | |||
|PK= | |||
|nonClinToxic='''Carcinogenesis, Mutagenesis, Impairment of Fertility''' | |nonClinToxic='''Carcinogenesis, Mutagenesis, Impairment of Fertility''' | ||
* Data on carcinogenesis, mutagenesis, and impairment of fertility are not available. | * Data on carcinogenesis, mutagenesis, and impairment of fertility are not available. | ||
|clinicalStudies=* There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
|howSupplied=* Capsules SYPRINE, 250 mg, are light brown opaque capsules coded SYPRINE on one side and ATON 710 on the other. They are supplied as follows: | |howSupplied=* Capsules SYPRINE, 250 mg, are light brown opaque capsules coded SYPRINE on one side and ATON 710 on the other. They are supplied as follows: | ||
| Line 319: | Line 96: | ||
* Store at 2-8°C (36-46°F). | * Store at 2-8°C (36-46°F). | ||
|fdaPatientInfo=* There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
|alcohol=* Alcohol-Trientine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-Trientine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
| | |brandNames=* SYPRINE ®<ref>{{Cite web | title =SYPRINE- trientine hydrochloride capsule| url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c34f77a7-996b-4470-b5df-d946a7fe5dbe }}</ref> | ||
|nlmPatientInfo=(Link to patient information page) | |nlmPatientInfo=(Link to patient information page) | ||
|drugShortage=Drug Shortage | |drugShortage=Drug Shortage | ||
}} | }} | ||
{{LabelImage | |||
|fileName=Syprine image1.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Syprine ingredients and appearance.png | |||
}} | |||
[[Category:Drug]] | |||
Latest revision as of 17:20, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Trientine is a heavy metal chelator that is FDA approved for the treatment of Wilson's disease. Common adverse reactions include iron deficiency, systemic lupus erythematosus,dystonia, muscular spasm, myasthenia gravis..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- SYPRINE is indicated in the treatment of patients with Wilson's disease who are intolerant of penicillamine. Clinical experience with SYPRINE is limited and alternate dosing regimens have not been well-characterized; all endpoints in determining an individual patient's dose have not been well defined. SYPRINE and penicillamine cannot be considered interchangeable. SYPRINE should be used when continued treatment with penicillamine is no longer possible because of intolerable or life endangering side effects.
- Unlike penicillamine, SYPRINE is not recommended in cystinuria or rheumatoid arthritis. The absence of a sulfhydryl moiety renders it incapable of binding cystine and, therefore, it is of no use in cystinuria. In 15 patients with rheumatoid arthritis, SYPRINE was reported not to be effective in improving any clinical or biochemical parameter after 12 weeks of treatment.
- SYPRINE is not indicated for treatment of biliary cirrhosis.
Dosage
- Systemic evaluation of dose and/or interval between dose has not been done. However, on limited clinical experience, the recommended initial dose of SYPRINE is 500-750 mg/day for pediatric patients and 750-1250 mg/day for adults given in divided doses two, three or four times daily. This may be increased to a maximum of 2000 mg/day for adults or 1500 mg/day for pediatric patients age 12 or under.
- The daily dose of SYPRINE should be increased only when the clinical response is not adequate or the concentration of free serum copper is persistently above 20 mcg/dL. Optimal long-term maintenance dosage should be determined at 6-12 month intervals.
- It is important that SYPRINE be given on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Trientine in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Trientine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- SYPRINE is indicated in the treatment of patients with Wilson's disease who are intolerant of penicillamine. Clinical experience with SYPRINE is limited and alternate dosing regimens have not been well-characterized; all endpoints in determining an individual patient's dose have not been well defined. SYPRINE and penicillamine cannot be considered interchangeable. SYPRINE should be used when continued treatment with penicillamine is no longer possible because of intolerable or life endangering side effects.
- Unlike penicillamine, SYPRINE is not recommended in cystinuria or rheumatoid arthritis. The absence of a sulfhydryl moiety renders it incapable of binding cystine and, therefore, it is of no use in cystinuria. In 15 patients with rheumatoid arthritis, SYPRINE was reported not to be effective in improving any clinical or biochemical parameter after 12 weeks of treatment.
- SYPRINE is not indicated for treatment of biliary cirrhosis.
Dosage
- Systemic evaluation of dose and/or interval between dose has not been done. However, on limited clinical experience, the recommended initial dose of SYPRINE is 500-750 mg/day for pediatric patients and 750-1250 mg/day for adults given in divided doses two, three or four times daily. This may be increased to a maximum of 2000 mg/day for adults or 1500 mg/day for pediatric patients age 12 or under.
- The daily dose of SYPRINE should be increased only when the clinical response is not adequate or the concentration of free serum copper is persistently above 20 mcg/dL. Optimal long-term maintenance dosage should be determined at 6-12 month intervals.
- It is important that SYPRINE be given on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Trientine in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Trientine in pediatric patients.
Contraindications
- Hypersensitivity to this product.
Warnings
- Patient experience with trientine hydrochloride is limited. Patients receiving SYPRINE should remain under regular medical supervision throughout the period of drug administration. Patients (especially women) should be closely monitored for evidence of iron deficiency anemia.
Adverse Reactions
Clinical Trials Experience
- Clinical experience with SYPRINE has been limited. The following adverse reactions have been reported in a clinical study in patients with Wilson's disease who were on therapy with trientine hydrochloride: iron deficiency, systemic lupus erythematosus. In addition, the following adverse reactions have been reported in marketed use: dystonia, muscular spasm, myasthenia gravis.
- SYPRINE is not indicated for treatment of biliary cirrhosis, but in one study of 4 patients treated with trientine hydrochloride for primary biliary cirrhosis, the following adverse reactions were reported: heartburn; epigastric pain and tenderness; thickening, fissuring and flaking of the skin; hypochromic microcytic anemia; acute gastritis; aphthoid ulcers; abdominal pain; melena; anorexia; malaise; cramps; muscle pain; weakness; rhabdomyolysis. A causal relationship of these reactions to drug therapy could not be rejected or established.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Trientine in the drug label.
Drug Interactions
- In general, mineral supplements should not be given since they may block the absorption of SYPRINE. However, iron deficiency may develop, especially in children and menstruating or pregnant women, or as a result of the low copper diet recommended for Wilson's disease. If necessary, iron may be given in short courses, but since iron and SYPRINE each inhibit absorption of the other, two hours should elapse between administration of SYPRINE and iron.
- It is important that SYPRINE be taken on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. This permits maximum absorption and reduces the likelihood of inactivation of the drug by metal binding in the gastrointestinal tract.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category C
- Trientine hydrochloride was teratogenic in rats at doses similar to the human dose. The frequencies of both resorptions and fetal abnormalities, including hemorrhage and edema, increased while fetal copper levels decreased when trientine hydrochloride was given in the maternal diets of rats. There are no adequate and well-controlled studies in pregnant women. SYPRINE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Trientine in women who are pregnant.
Labor and Delivery
- There is no FDA guidance on use of Trientine during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SYPRINE is administered to a nursing mother.
Pediatric Use
- Controlled studies of the safety and effectiveness of SYPRINE in pediatric patients have not been conducted. It has been used clinically in pediatric patients as young as 6 years with no reported adverse experiences.
Geriatic Use
- Clinical studies of SYPRINE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience is insufficient to determine differences in responses between the elderly and younger patients. In general, dose selection should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Gender
- There is no FDA guidance on the use of Trientine with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Trientine with respect to specific racial populations.
Renal Impairment
- There is no FDA guidance on the use of Trientine in patients with renal impairment.
Hepatic Impairment
- There is no FDA guidance on the use of Trientine in patients with hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Trientine in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Trientine in patients who are immunocompromised.
Others
Administration and Monitoring
Administration
- Oral
- Patients should be directed to take SYPRINE on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed. Because of the potential for contact dermatitis, any site of exposure to the capsule contents should be washed with water promptly. For the first month of treatment, the patient should have his temperature taken nightly, and he should be asked to report any symptom such as fever or skin eruption.
Monitoring
- There is limited information regarding Monitoring of Trientine in the drug label.
IV Compatibility
- There is limited information regarding IV Compatibility of Trientine in the drug label.
Overdosage
Acute Overdose
- There is a report of an adult woman who ingested 30 grams of trientine hydrochloride without apparent ill effects. No other data on overdosage are available.
Pharmacology
Mechanism of Action
- There is limited information regarding Mechanism Of Action of Trientine in the drug label.
Structure
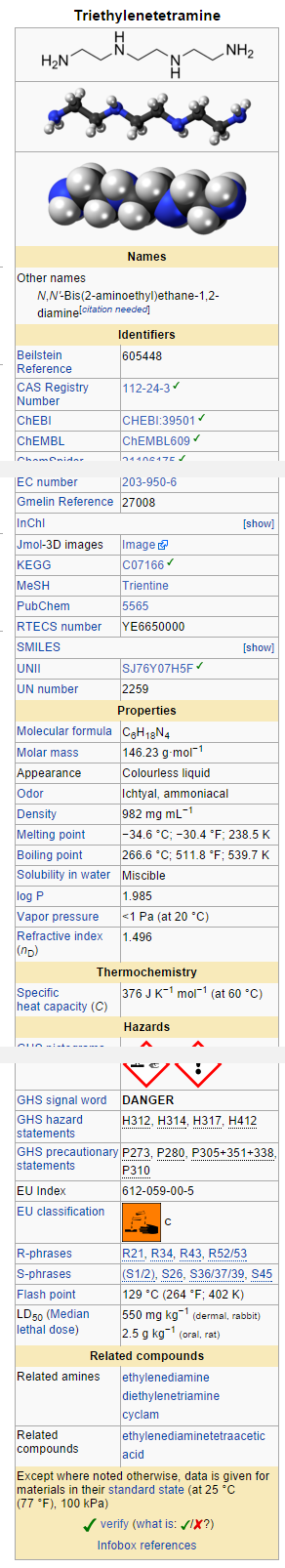
- Trientine hydrochloride is N,N'-bis (2-aminoethyl)-1,2-ethanediamine dihydrochloride. It is a white to pale yellow crystalline hygroscopic powder. It is freely soluble in water, soluble in methanol, slightly soluble in ethanol, and insoluble in chloroform and ether.
The empirical formula is C6H18N4•2HCl with a molecular weight of 219.2. The structural formula is:
NH2(CH2)2NH(CH2)2NH(CH2)2NH2•2HCl
Trientine hydrochloride is a chelating compound for removal of excess copper from the body. SYPRINE1 (Trientine Hydrochloride) is available as 250 mg capsules for oral administration. Capsules SYPRINE contain gelatin, iron oxides, stearic acid, and titanium dioxide as inactive ingredients.
Pharmacodynamics
- There is limited information regarding Pharmacodynamics of Trientine in the drug label.
Pharmacokinetics
- Data on the pharmacokinetics of trientine hydrochloride are not available. Dosage adjustment recommendations are based upon clinical use of the drug.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Data on carcinogenesis, mutagenesis, and impairment of fertility are not available.
Clinical Studies
- There is limited information regarding Clinical Studies of Trientine in the drug label.
How Supplied
- Capsules SYPRINE, 250 mg, are light brown opaque capsules coded SYPRINE on one side and ATON 710 on the other. They are supplied as follows:
- NDC 0187-2120-10 in bottles of 100.
Storage
- Keep container tightly closed.
- Store at 2-8°C (36-46°F).
Images
Drug Images
{{#ask: Page Name::Trientine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Trientine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- There is limited information regarding Patient Counseling Information of Trientine in the drug label.
Precautions with Alcohol
- Alcohol-Trientine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- SYPRINE ®[1]
Look-Alike Drug Names
There is limited information regarding Trientine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Trientine |Label Name=Syprine image1.jpg
}}
{{#subobject:
|Label Page=Trientine |Label Name=Syprine ingredients and appearance.png
}}