Trandolapril description: Difference between revisions
Amr Marawan (talk | contribs) (Created page with "__NOTOC__ {{Trandolapril}} {{CMG}}; {{AE}} {{AM}} <ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = MAVIK (TRANDOLAPRIL) TABLET [ABBVIE INC.] | url ...") |
Amr Marawan (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
{{CMG}}; {{AE}} {{AM}} | {{CMG}}; {{AE}} {{AM}} | ||
<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = MAVIK (TRANDOLAPRIL) TABLET [ABBVIE INC.] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ad67ea2-409c-4f52-a9b8-38216209609a | publisher = | date = | accessdate = }}</ref> | ==DESCRIPTION== | ||
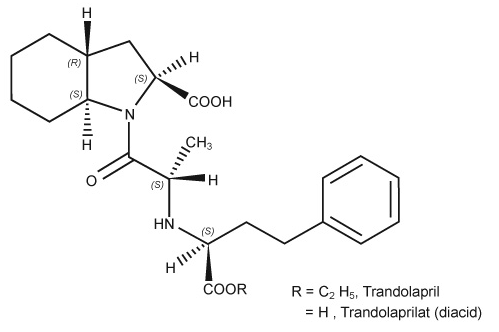
Trandolapril is the ethyl ester prodrug of a nonsulfhydryl angiotensin converting enzyme (ACE) inhibitor, trandolaprilat. Trandolapril is chemically described as (2S, 3aR, 7aS)-1-[(S)-N-[(S)-1-Carboxy-3-phenylpropyl]alanyl] hexahydro-2-indolinecarboxylic acid, 1-ethyl ester. Its empirical formula is C24H34N2O5 and its structural formula is | |||
{| | |||
| [[File:dd6.png|800px|thumb]] | |||
|} | |||
M.W. = 430.54 | |||
Melting Point = 125°C | |||
Trandolapril is a white or almost white powder that is soluble (> 100 mg/mL) in chloroform, dichloromethane, and methanol. MAVIK tablets contain 1 mg, 2 mg, or 4 mg of trandolapril for oral administration. Each tablet also contains corn starch, croscarmellose sodium, hypromellose, iron oxide, lactose monohydrate, povidone, sodium stearyl fumarate.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = MAVIK (TRANDOLAPRIL) TABLET [ABBVIE INC.] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ad67ea2-409c-4f52-a9b8-38216209609a | publisher = | date = | accessdate = }}</ref> | |||
==References== | ==References== | ||
Revision as of 21:59, 18 February 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Amr Marawan, M.D. [2]
DESCRIPTION
Trandolapril is the ethyl ester prodrug of a nonsulfhydryl angiotensin converting enzyme (ACE) inhibitor, trandolaprilat. Trandolapril is chemically described as (2S, 3aR, 7aS)-1-[(S)-N-[(S)-1-Carboxy-3-phenylpropyl]alanyl] hexahydro-2-indolinecarboxylic acid, 1-ethyl ester. Its empirical formula is C24H34N2O5 and its structural formula is
 |
M.W. = 430.54
Melting Point = 125°C
Trandolapril is a white or almost white powder that is soluble (> 100 mg/mL) in chloroform, dichloromethane, and methanol. MAVIK tablets contain 1 mg, 2 mg, or 4 mg of trandolapril for oral administration. Each tablet also contains corn starch, croscarmellose sodium, hypromellose, iron oxide, lactose monohydrate, povidone, sodium stearyl fumarate.[1]
References
Adapted from the FDA Package Insert.