Trandolapril adverse reactions: Difference between revisions
Amr Marawan (talk | contribs) No edit summary |
Amr Marawan (talk | contribs) No edit summary |
||
| Line 5: | Line 5: | ||
==Adverse Reactions== | ==Adverse Reactions== | ||
The safety experience in U.S. placebo-controlled trials included 1069 hypertensive patients, of whom 832 received MAVIK. Nearly 200 hypertensive patients received MAVIK for over one year in open-label trials. In controlled trials, withdrawals for adverse events were 2.1% on placebo and 1.4% on MAVIK. Adverse events considered at least possibly related to treatment occurring in 1% of MAVIK-treated patients and more common on MAVIK than placebo, pooled for all doses, are shown below, together with the frequency of discontinuation of treatment because of these events. | The safety experience in U.S. placebo-controlled trials included 1069 hypertensive patients, of whom 832 received MAVIK. Nearly 200 hypertensive patients received MAVIK for over one year in open-label trials. In controlled trials, withdrawals for adverse events were 2.1% on placebo and 1.4% on MAVIK. Adverse events considered at least possibly related to treatment occurring in 1% of MAVIK-treated patients and more common on MAVIK than placebo, pooled for all doses, are shown below, together with the frequency of discontinuation of treatment because of these events. | ||
{| | {| | ||
| [[File:dd1.png|800px|thumb]] | | [[File:dd1.png|800px|thumb]] | ||
|} | |} | ||
[[Headache]] and [[fatigue]] were all seen in more than 1% of MAVIK-treated patients but were more frequently seen on placebo. Adverse events were not usually persistent or difficult to manage. | [[Headache]] and [[fatigue]] were all seen in more than 1% of MAVIK-treated patients but were more frequently seen on placebo. Adverse events were not usually persistent or difficult to manage. | ||
====Left Ventricular Dysfunction Post Myocardial Infarction==== | ====Left Ventricular Dysfunction Post Myocardial Infarction==== | ||
| Line 18: | Line 16: | ||
|} | |} | ||
Clinical adverse experiences possibly or probably related or of uncertain relationship to therapy occurring in 0.3% to 1.0% (except as noted) of the patients treated with MAVIK (with or without concomitant calcium ion antagonist or diuretic) in controlled or uncontrolled trials (N=1134) and less frequent, clinically significant events seen in clinical trials or post-marketing experience include (listed by body system): | Clinical adverse experiences possibly or probably related or of uncertain relationship to therapy occurring in 0.3% to 1.0% (except as noted) of the patients treated with MAVIK (with or without concomitant calcium ion antagonist or diuretic) in controlled or uncontrolled trials (N=1134) and less frequent, clinically significant events seen in clinical trials or post-marketing experience include (listed by body system): | ||
====General Body Function==== | ====General Body Function==== | ||
[[Chest pain]]. | [[Chest pain]]. | ||
====Cardiovascular==== | ====Cardiovascular==== | ||
[[AV first degree block]], [[bradycardia]], [[edema]], [[flushing]], and [[palpitations]]. | [[AV first degree block]], [[bradycardia]], [[edema]], [[flushing]], and [[palpitations]]. | ||
====Central Nervous System==== | ====Central Nervous System==== | ||
[[Drowsiness]], [[insomnia]], [[paresthesia]], [[vertigo]]. | [[Drowsiness]], [[insomnia]], [[paresthesia]], [[vertigo]]. | ||
====Dermatologic==== | ====Dermatologic==== | ||
| Line 43: | Line 37: | ||
====Gastrointestinal==== | ====Gastrointestinal==== | ||
[[Abdominal distention]], [[abdominal pain]]/[[cramps]], [[constipation]], [[dyspepsia]], [[diarrhea]], [[vomiting]], [[nausea]]. | [[Abdominal distention]], [[abdominal pain]]/[[cramps]], [[constipation]], [[dyspepsia]], [[diarrhea]], [[vomiting]], [[nausea]]. | ||
====Hemopoietic==== | ====Hemopoietic==== | ||
Decreased leukocytes, decreased neutrophils. | Decreased leukocytes, decreased neutrophils. | ||
====Metabolism and Endocrine==== | ====Metabolism and Endocrine==== | ||
| Line 57: | Line 49: | ||
====Pulmonary==== | ====Pulmonary==== | ||
[[Dyspnea]]. | [[Dyspnea]]. | ||
====Postmarketing==== | ====Postmarketing==== | ||
| Line 67: | Line 58: | ||
====Cardiovascular==== | ====Cardiovascular==== | ||
[[Myocardial infarction]], [[myocardial ischemia]], [[angina pectoris]], [[cardiac failure]], [[ventricular tachycardia]], tachycardia, [[transient ischemic attack]], [[arrhythmia]]. | [[Myocardial infarction]], [[myocardial ischemia]], [[angina pectoris]], [[cardiac failure]], [[ventricular tachycardia]], tachycardia, [[transient ischemic attack]], [[arrhythmia]]. | ||
====Central Nervous System==== | ====Central Nervous System==== | ||
[[Cerebral hemorrhage]]. | [[Cerebral hemorrhage]]. | ||
====Dermatologic==== | ====Dermatologic==== | ||
| Line 81: | Line 70: | ||
====Gastrointestinal==== | ====Gastrointestinal==== | ||
[[Dry mouth]], [[pancreatitis]], [[jaundice]] and [[hepatitis]]. | [[Dry mouth]], [[pancreatitis]], [[jaundice]] and [[hepatitis]]. | ||
====Hemopoietic==== | ====Hemopoietic==== | ||
| Line 88: | Line 76: | ||
====Metabolism and Endocrine==== | ====Metabolism and Endocrine==== | ||
Increased [[SGOT]] (AST). | Increased [[SGOT]] (AST). | ||
====Pulmonary==== | ====Pulmonary==== | ||
| Line 100: | Line 87: | ||
====Hematology==== | ====Hematology==== | ||
[[Thrombocytopenia]]. | [[Thrombocytopenia]]. | ||
====Serum Electrolytes==== | ====Serum Electrolytes==== | ||
[[Hyponatremia]]. | [[Hyponatremia]]. | ||
====Creatinine and Blood Urea Nitrogen==== | ====Creatinine and Blood Urea Nitrogen==== | ||
| Line 111: | Line 96: | ||
====Liver Function Tests==== | ====Liver Function Tests==== | ||
Occasional elevation of transaminases at the rate of 3X upper normals occurred in 0.8% of patients and persistent increase in [[bilirubin]] occurred in 0.2% of patients. Discontinuation for elevated liver enzymes occurred in 0.2% of patients. | Occasional elevation of transaminases at the rate of 3X upper normals occurred in 0.8% of patients and persistent increase in [[bilirubin]] occurred in 0.2% of patients. Discontinuation for elevated liver enzymes occurred in 0.2% of patients. | ||
====Other==== | ====Other==== | ||
Revision as of 22:12, 18 February 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Amr Marawan, M.D. [2]
Adverse Reactions
The safety experience in U.S. placebo-controlled trials included 1069 hypertensive patients, of whom 832 received MAVIK. Nearly 200 hypertensive patients received MAVIK for over one year in open-label trials. In controlled trials, withdrawals for adverse events were 2.1% on placebo and 1.4% on MAVIK. Adverse events considered at least possibly related to treatment occurring in 1% of MAVIK-treated patients and more common on MAVIK than placebo, pooled for all doses, are shown below, together with the frequency of discontinuation of treatment because of these events.
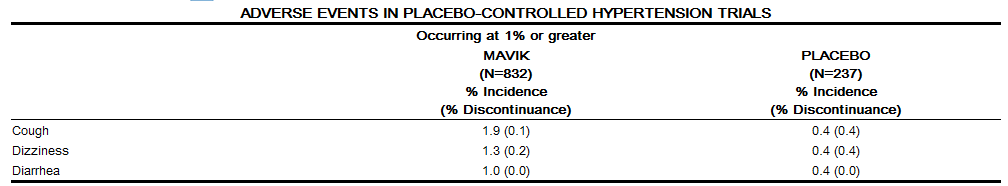 |
Headache and fatigue were all seen in more than 1% of MAVIK-treated patients but were more frequently seen on placebo. Adverse events were not usually persistent or difficult to manage.
Left Ventricular Dysfunction Post Myocardial Infarction
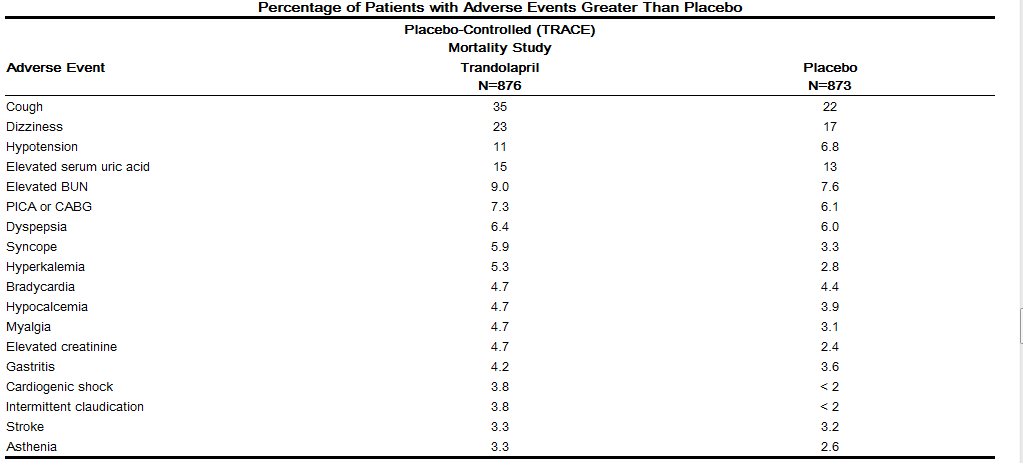
Adverse reactions related to MAVIK occurring at a rate greater than that observed in placebo-treated patients with left ventricular dysfunction, are shown below. The incidences represent the experiences from the TRACE study. The follow-up time was between 24 and 50 months for this study.
 |
Clinical adverse experiences possibly or probably related or of uncertain relationship to therapy occurring in 0.3% to 1.0% (except as noted) of the patients treated with MAVIK (with or without concomitant calcium ion antagonist or diuretic) in controlled or uncontrolled trials (N=1134) and less frequent, clinically significant events seen in clinical trials or post-marketing experience include (listed by body system):
General Body Function
Cardiovascular
AV first degree block, bradycardia, edema, flushing, and palpitations.
Central Nervous System
Drowsiness, insomnia, paresthesia, vertigo.
Dermatologic
Eye, Ear, Nose, Throat
Epistaxis, throat inflammation, upper respiratory tract infection.
Emotional, Mental, Sexual States
Anxiety, impotence, decreased libido.
Gastrointestinal
Abdominal distention, abdominal pain/cramps, constipation, dyspepsia, diarrhea, vomiting, nausea.
Hemopoietic
Decreased leukocytes, decreased neutrophils.
Metabolism and Endocrine
Increased liver enzymes including SGPT (ALT).
Musculoskeletal System
Extremity pain, muscle cramps, gout.
Pulmonary
Postmarketing
The following adverse reactions were identified during post approval use of MAVIK. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General Body Function
Cardiovascular
Myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, ventricular tachycardia, tachycardia, transient ischemic attack, arrhythmia.
Central Nervous System
Dermatologic
Alopecia, sweating, Stevens-Johnson syndrome and toxic epidermal necrolysis.
Emotional, Mental, Sexual States
Gastrointestinal
Dry mouth, pancreatitis, jaundice and hepatitis.
Hemopoietic
Agranulocytosis, pancytopenia.
Metabolism and Endocrine
Increased SGOT (AST).
Pulmonary
Renal and Urinary
Clinical Laboratory Test Findings
Hematology
Serum Electrolytes
Creatinine and Blood Urea Nitrogen
Increases in creatinine levels occurred in 1.1% of patients receiving MAVIK alone and 7.3% of patients treated with MAVIK, a calcium ion antagonist and a diuretic. Increases in blood urea nitrogen levels occurred in 0.6% of patients receiving MAVIK alone and 1.4% of patients receiving MAVIK, a calcium ion antagonist, and a diuretic. None of these increases required discontinuation of treatment. Increases in these laboratory values are more likely to occur in patients with renal insufficiency or those pretreated with a diuretic and, based on experience with other ACE inhibitors, would be expected to be especially likely in patients with renal artery stenosis.
Liver Function Tests
Occasional elevation of transaminases at the rate of 3X upper normals occurred in 0.8% of patients and persistent increase in bilirubin occurred in 0.2% of patients. Discontinuation for elevated liver enzymes occurred in 0.2% of patients.
Other
Another potentially important adverse experience, eosinophilic pneumonitis, has been attributed to other ACE inhibitors.[1]
References
Adapted from the FDA Package Insert.