Tobramycin (opthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tobramycin (opthalmic) is an antibiotic that is FDA approved for the treatment of external infections of the eye and its adnexa caused by susceptible bacteria. Common adverse reactions include lid itching, lid swelling, and conjunctival erythema.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Tobramycin Ophthalmic Solution is a topical antibiotic indicated in the treatment of external infections of the eye and its adnexa caused by susceptible bacteria. Appropriate monitoring of bacterial response to topical antibiotic therapy should accompany the use of Tobramycin Ophthalmic Solution. Clinical studies have shown tobramycin to be safe and effective for use in children.
Dosing Information
- In mild to moderate disease, instill one or two drops into the affected eye(s) every four hours. In severe infections, instill two drops into the eye(s) hourly until improvement, following which treatment should be reduced prior to discontinuation.
- DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT.
- FOR OPHTHALMIC USE ONLY.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tobramycin (opthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tobramycin (opthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Tobramycin (opthalmic) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tobramycin (opthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tobramycin (opthalmic) in pediatric patients.
Contraindications
- Tobramycin Ophthalmic Solution is contraindicated in patients with known hypersensitivity to any of its components.
Warnings
- NOT FOR INJECTION INTO THE EYE. Sensitivity to topically applied aminoglycosides may occur in some patients. If a sensitivity reaction to Tobramycin Ophthalmic Solution occurs, discontinue use.
PRECAUTIONS:
General:
- As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, appropriate therapy should be initiated.
Adverse Reactions
Clinical Trials Experience
- The most frequent adverse reactions to tobramycin ophthalmic solution is localized ocular toxicity and hypersensitivity, including lid itching and swelling, and conjunctival erythema. These reactions occur in less than three of 100 patients treated with tobramycin. Similar reactions may occur with the topical use of other aminoglycoside antibiotics. Other adverse reactions have not been reported from tobramycin therapy; however, if topical ocular tobramycin is administered concomitantly with systemic aminoglycoside antibiotics, care should be taken to monitor the total serum concentration.
Postmarketing Experience
There is limited information regarding Tobramycin (opthalmic) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Tobramycin (opthalmic) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies in three types of animals at doses up to thirty-three times the normal human systemic dose have revealed no evidence of impaired fertility or harm to the fetus due to tobramycin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tobramycin (opthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tobramycin (opthalmic) during labor and delivery.
Nursing Mothers
- Because of the potential for adverse reactions in nursing infants from Tobramycin Ophthalmic Solution, a decision should be made whether to discontinue nursing the infant or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Tobramycin (opthalmic) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Tobramycin (opthalmic) in geriatric settings.
Gender
There is no FDA guidance on the use of Tobramycin (opthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tobramycin (opthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tobramycin (opthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tobramycin (opthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tobramycin (opthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tobramycin (opthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic solution
Monitoring
There is limited information regarding Tobramycin (opthalmic) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tobramycin (opthalmic) and IV administrations.
Overdosage
- Clinically apparent signs and symptoms of an overdose of tobramycin ophthalmic solution (punctate keratitis, erythema, increased lacrimation, edema and lid itching) may be similar to adverse reaction effects seen in some patients.
Pharmacology
Mechanism of Action
There is limited information regarding Tobramycin (opthalmic) Mechanism of Action in the drug label.
Structure
- Tobramycin ophthalmic solution is a sterile topical ophthalmic antibiotic formulation prepared specifically for topical therapy of external infections.
EACH mL CONTAINS:
- ACTIVE: Tobramycin 3 mg (0.3%). INACTIVES: Boric Acid, Sodium Sulfate, Sodium Chloride, Tyloxapol and Purified Water. Sodium Hydroxide and/or Sulfuric Acid may be added to adjust pH (7.0 - 8.0).
- PRESERVATIVE ADDED: Benzalkonium Chloride 0.1 mg (0.01%).
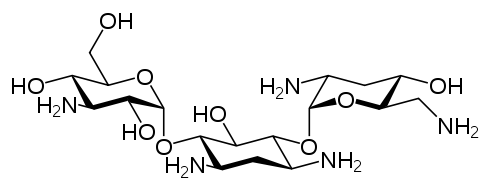
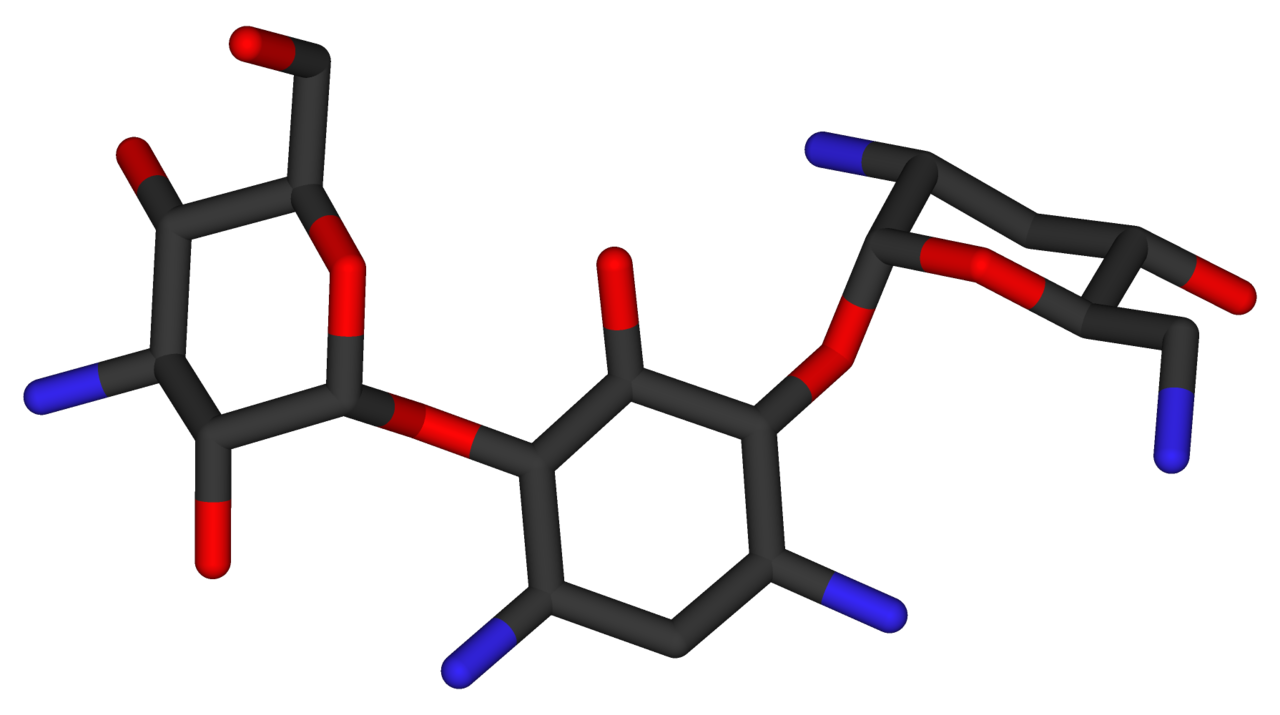
- The structural formula of tobramycin is

- Molecular formula: C18H37N5O9
- Molecular weight: 467.52
- Chemical name:
- O-[3-amino-3-deoxy—α-D-gluco-pyranosyl-(1 → 4)]-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribohexo-pyranosyl- (1 → 6)]-2-deoxystreptamine.
Pharmacodynamics
In Vitro Data:
- In vitro studies have demonstrated tobramycin is active against susceptible strains of the following microorganisms:
- Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
- Streptococci, including some of the Group A-betahemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
- Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, and Acinetobacter calcoaceticus and some Neisseria species. Bacterial susceptibility studies demonstrate that in some cases, microorganisms resistant to gentamicin retain susceptibility to tobramycin. A significant bacterial population resistant to tobramycin has not yet emerged; however, bacterial resistance may develop upon prolonged use.
Pharmacokinetics
There is limited information regarding Tobramycin (opthalmic) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Tobramycin (opthalmic) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Tobramycin (opthalmic) Clinical Studies in the drug label.
How Supplied
Tobramycin Ophthalmic Solution USP, 0.3% is supplied in a plastic bottle with a controlled drop tip in the following size:
5 mL bottle - Prod. No. 24207
Revised August 2007
Bausch & Lomb Incorporated Tampa, FL 33637 ©Bausch & Lomb Incorporated
9116801 (Folded) 9116901 (Flat)
Storage
- Store at 2°-25°C (36°-77°F). Avoid excessive heat.
- KEEP OUT OF REACH OF CHILDREN.
Images
Drug Images
{{#ask: Page Name::Tobramycin (opthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
NDC 24208-290-05
Bausch & Lomb
Tobramycin Ophthalmic Solution USP, 0.3% (Sterile)
Rx only
[icon- eye] [icon- 0.3%] [icon- solution] [icon- 5 mL]


{{#ask: Label Page::Tobramycin (opthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Do not touch dropper tip to any surface, as this may contaminate the contents.
- DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT.
- FOR OPHTHALMIC USE ONLY
Precautions with Alcohol
Alcohol-Tobramycin (opthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Tobramycin (opthalmic) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Tobramycin (opthalmic) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.