Thoracic aortic aneurysm pathophysiology
|
Thoracic aortic aneurysm Microchapters |
|
Differentiating Thoracic Aortic Aneurysm from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Special Scenarios |
|
Case Studies |
|
Thoracic aortic aneurysm pathophysiology On the Web |
|
Directions to Hospitals Treating Thoracic aortic aneurysm pathophysiology |
|
Risk calculators and risk factors for Thoracic aortic aneurysm pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aarti Narayan, M.B.B.S [2]
Overview
An aneurysm occurs when a part or entire circumference of the vessel is pathologically dilated. A true aneurysm involves all three layers of the vessel, whereas pseudoaneurysm is characterized by disruption of the intima and media, and the dilated segment of the aorta is lined by adventitia alone. Thoracic aortic aneurysm (TAA) is a clinically silent and potentially fatal disease whose pathophysiology is poorly understood. Application of data derived from animal models and human tissue analysis of abdominal aortic aneurysms may prove misleading given current evidence of structural and biochemical aortic heterogeneity above and below the diaphragm. Genetic predisposition is more common in TAA and includes multi-faceted syndromes such as Marfan, Loeys-Dietz, and type IV Ehlers-Danlos as well as autosomal-dominant familial patterns of inheritance. Investigation into the consequences of these known mutations has provided insight into the cell signaling cascades leading to degenerative remodeling of the aortic medial extracellular matrix (ECM) with TGF-β playing a major role. Targeted research into modifying the upstream regulation or downstream effects of the TGF-β1 pathway may provide opportunities for intervention to attenuate TAA progression.
Pathophysiology
- The clinical manifestations of thoracic aortic aneurysms depends on hemo-dynamic factors as well as factors intrinsic to individual arterial components.
- Cystic medial necrosis is the most common pathology associated with ascending aortic aneurysms, whereas atherosclerosis is most frequently involved in the arch and descending aorta.
- The aortic aneurysms associated with marfan syndrome grow at a faster rate and are more prone to rupture.
- Most thoracic aneurysms are asymptomatic. However, the enlarging aorta can compress adjacent organs and cause symptoms like chest pain, dyspnea, hoarseness of voice, cough and dysphagia. Also symptoms of congestive heart failure can occur from severe aortic regurgitation and congestion of head, neck and upper extremities from superior vena cava compression.[1][2]
Elastin and Collagen
- Increased activity of certain enzymes causes degradation of elastin and collagen in the arteries. This leads to loss of elasticity, weakens the aortic wall and causes it to dilate.[3]
- Moreover, the part of aorta below the origin of renal arteries has less amount of elastin compared to collagen, accounting for increased frequency of aneurysms in this area[4].
- In patients with marfan's syndrome there is a defect on chromosome 15 that is associated with impaired fibrillin synthesis, which is the core protein of microfibrils. Frequently among patients with marfan's syndrome there is cystic medial degeneration[5].
Hemodynamic Factors
- The aorta being a low resistance circuit, repeated trauma from a reflected wave results in dilatation[6].
- Systemic hypertension accelerates the process of dilatation of aorta and contributes to the formation of aneurysms[7][8][9].
Genetics
- FBN1: This is one of the genes involved in marfan's syndrome[10][11].
- TGFBR1: This is one of the genes involved in loeys-dietz syndrome.
- TGFBR2: This is one of the genes involved in loeys-dietz syndrome.
- COL3A1: This is one of the genes involved in ehlers danlos syndrome.
- ACTA2: This is one of the genes involved in familial thoracic aortic aneurysm and dissection (FTAAD).
Pathogenesis
- During embryologic development, the ascending and arch aorta to the ligamentum arteriosum formed by cells from the neural crest, and the media.
- It grows by assembling sequential lamellar units to reach a total of 55-60 by adulthood.
- It always maintaining a constant ratio of aortic diameter to medial thickness.
- Interestingly, that ratio remains consistent during abdominal aortic growth as well, but in that region the precursor cells originate in the mesoderm and the number of lamellar units remains constant while the thickness of each unit is expanded during maturation.
- This disparity in aortic cellular origin and segmental growth patterns contributes to differences in and extracellular matrix (ECM) microfibril density and VSMC reactivity to vasoactive growth factors , with downstream effects on susceptibility to aneurysmal degeneration.
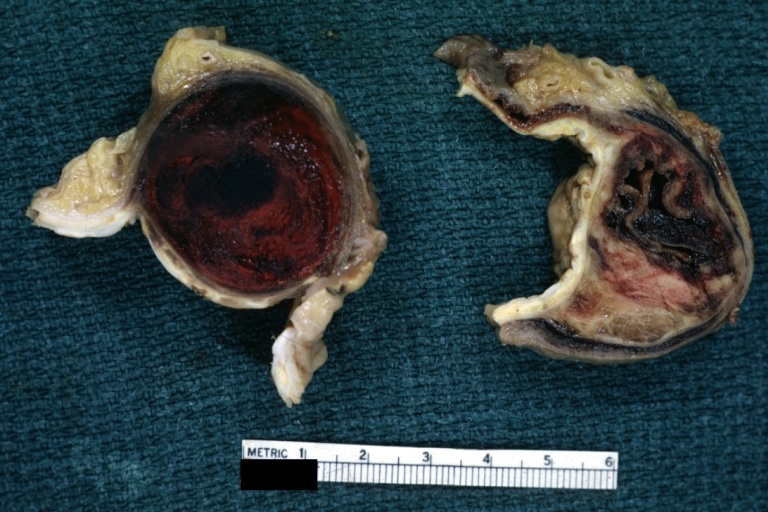
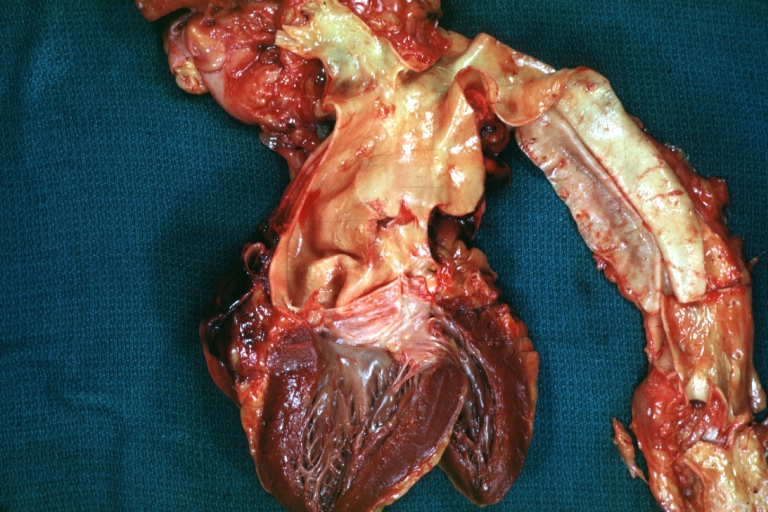
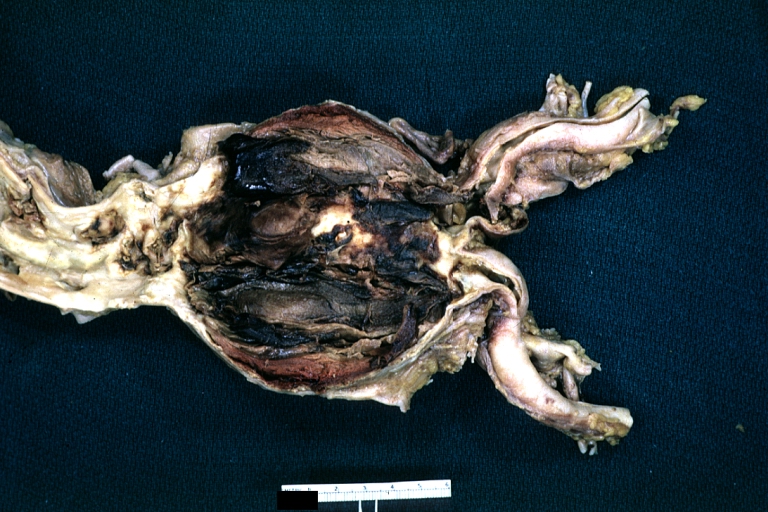
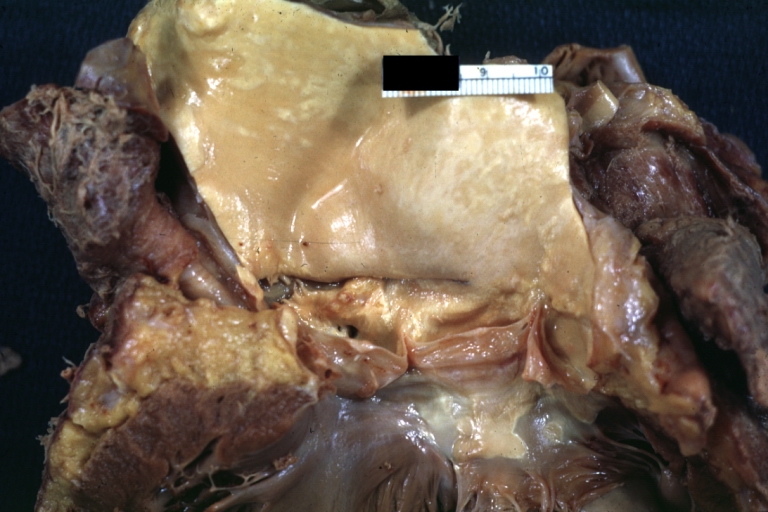
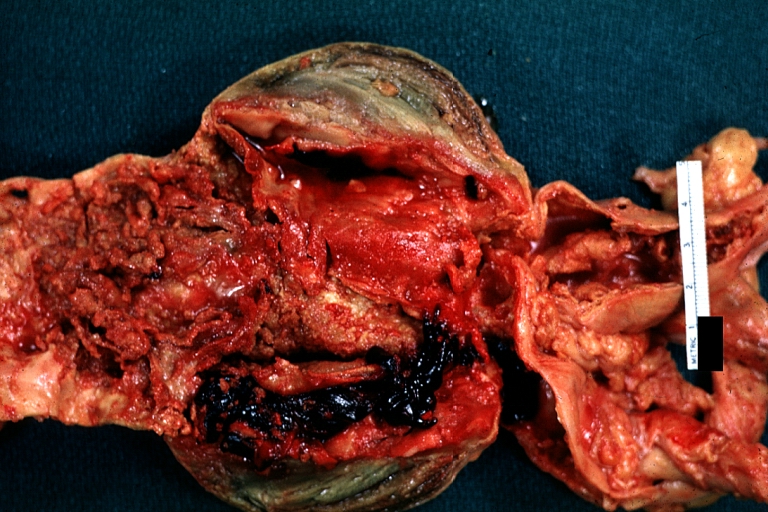
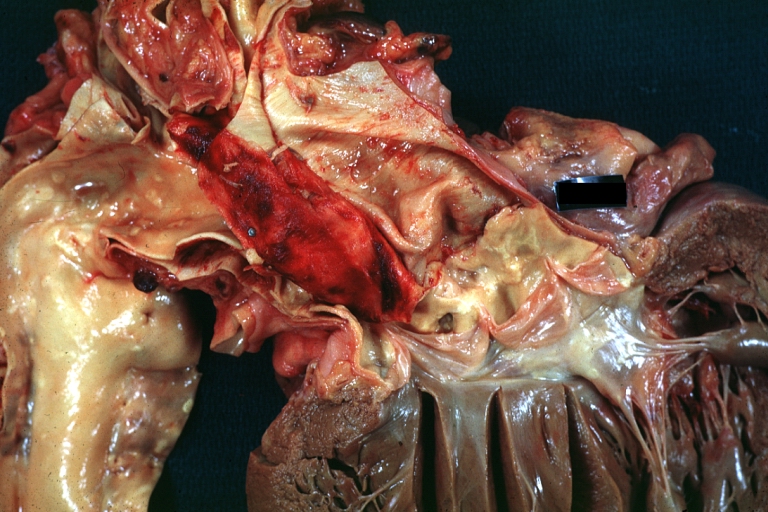
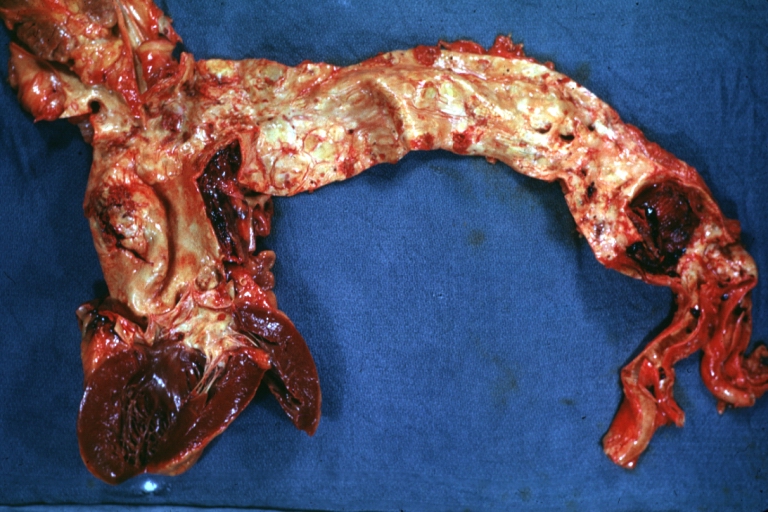
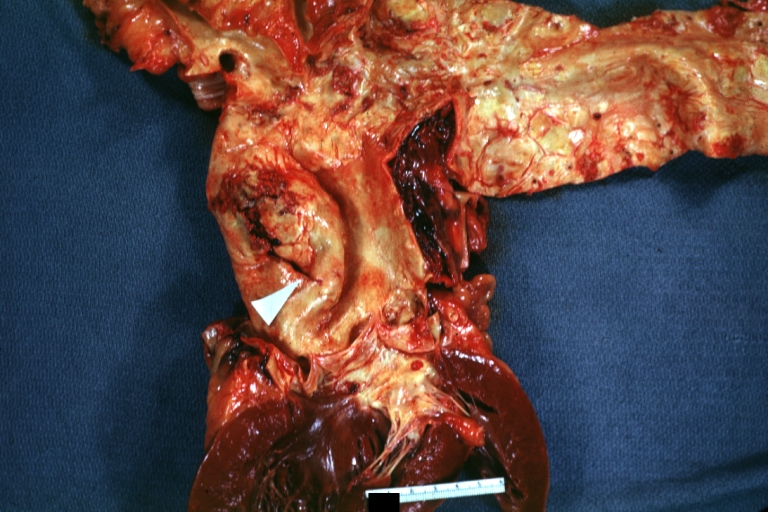
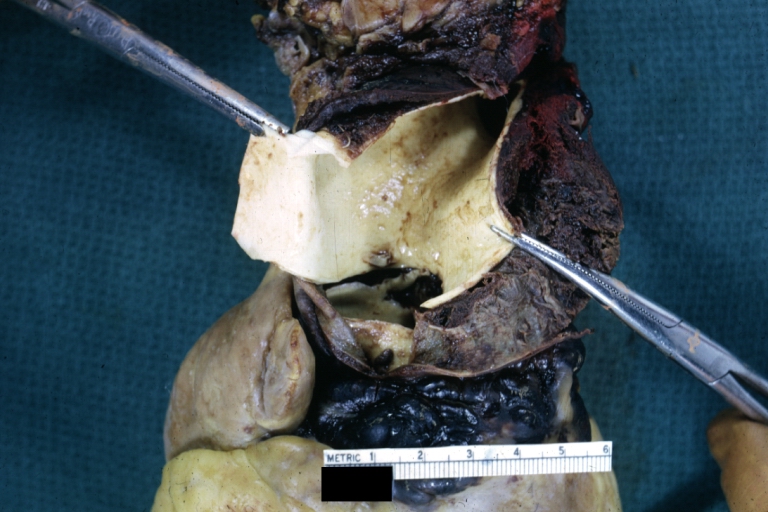
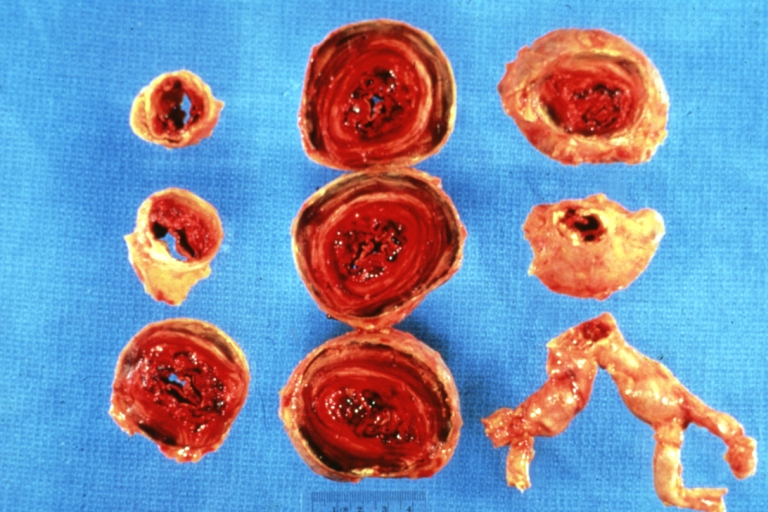
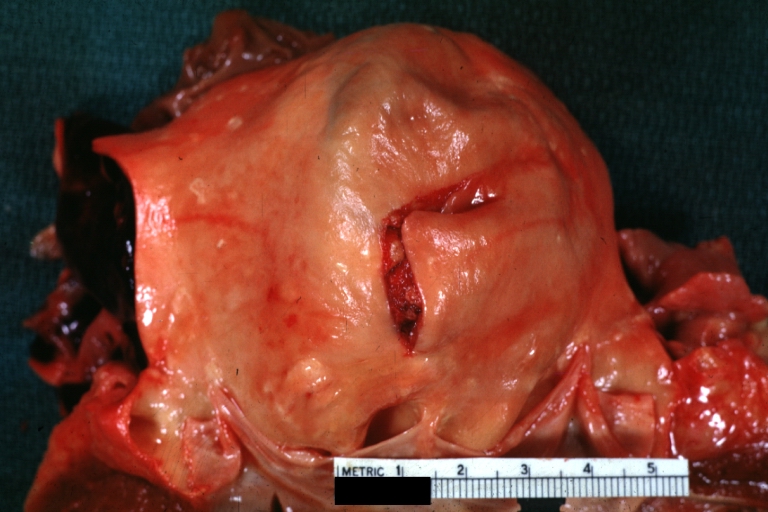
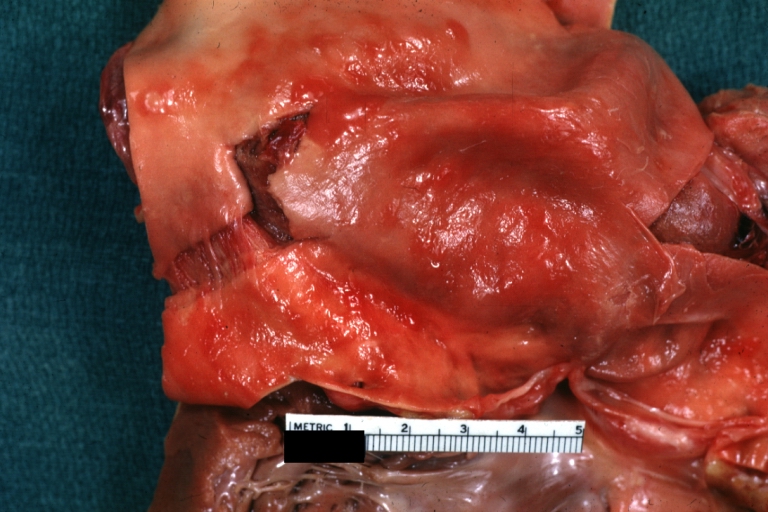
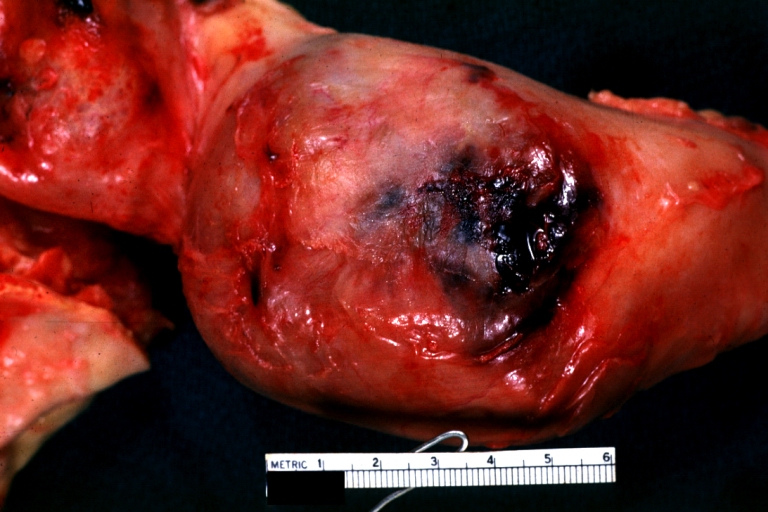
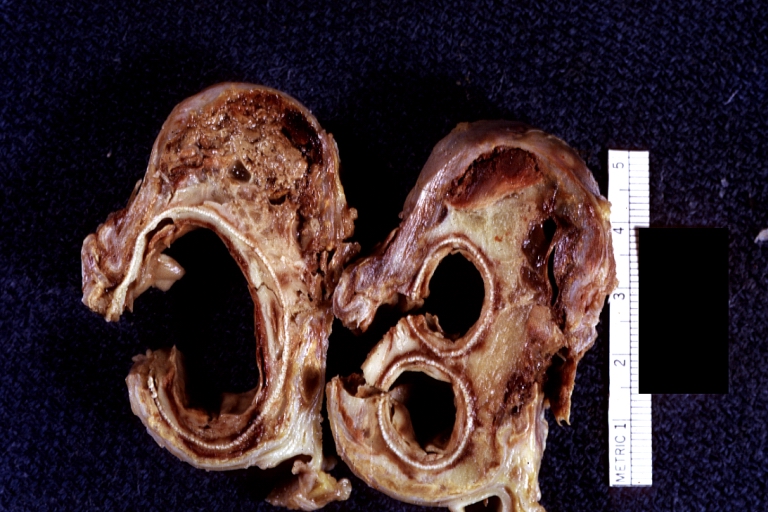
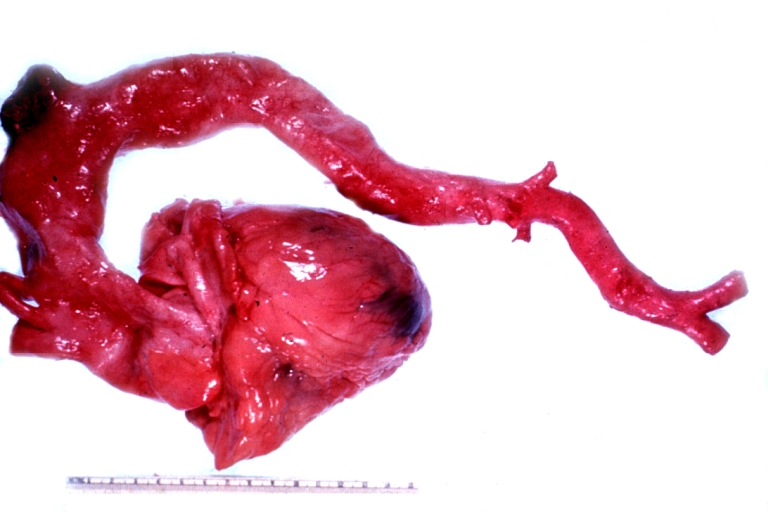
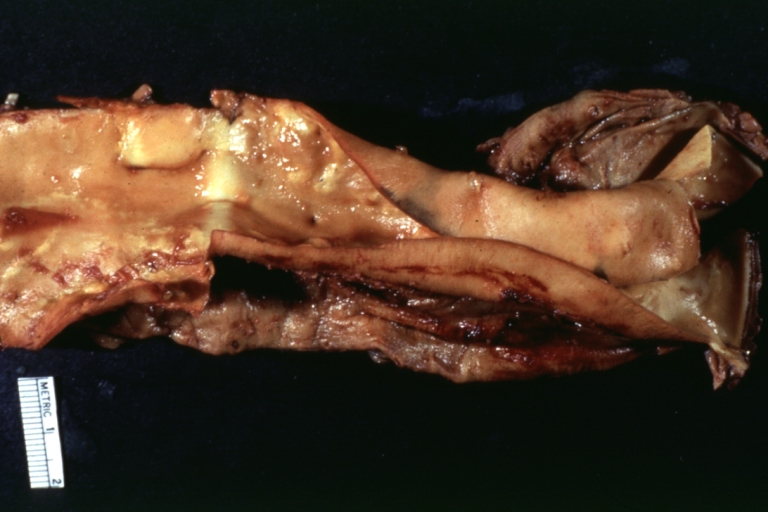
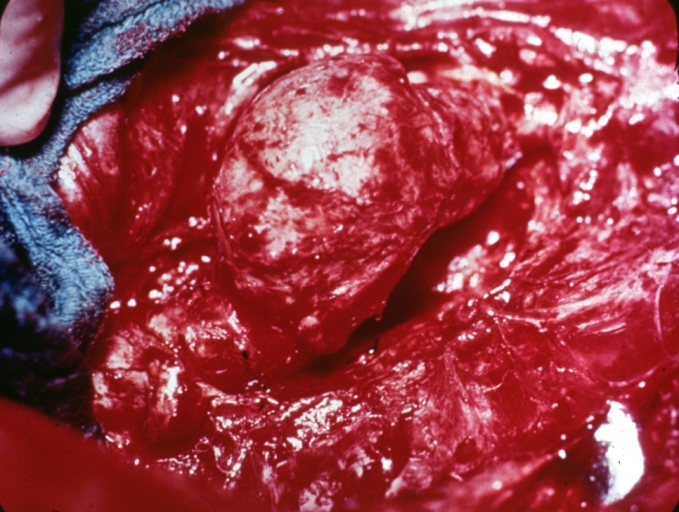
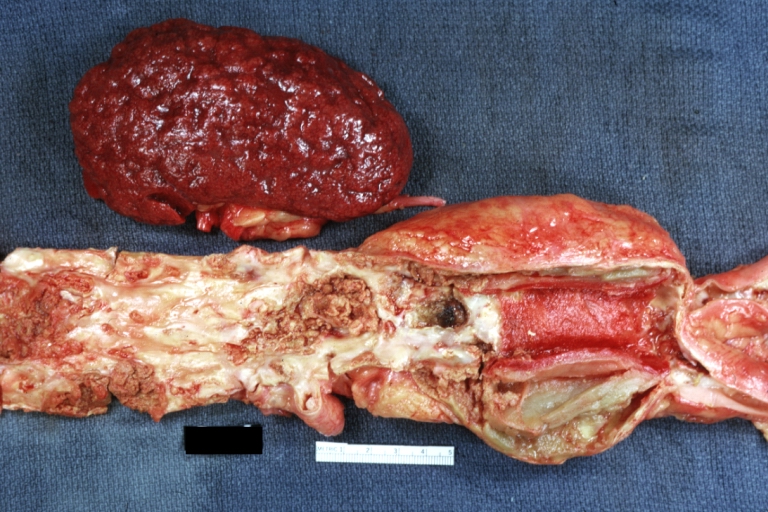
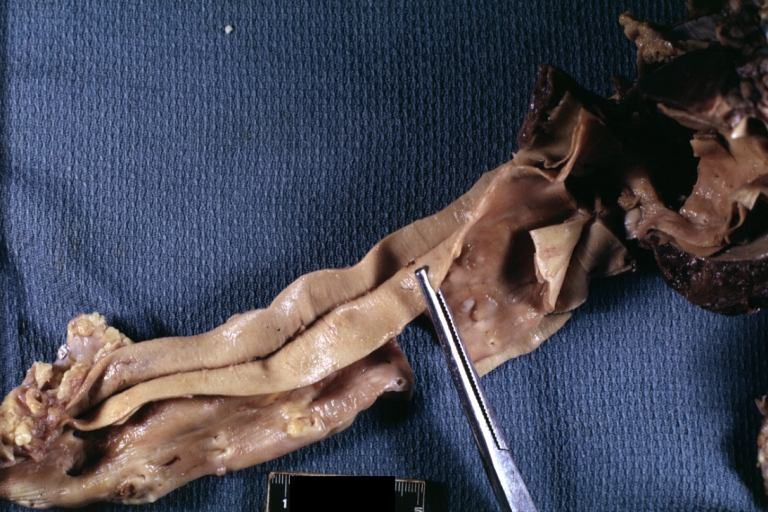
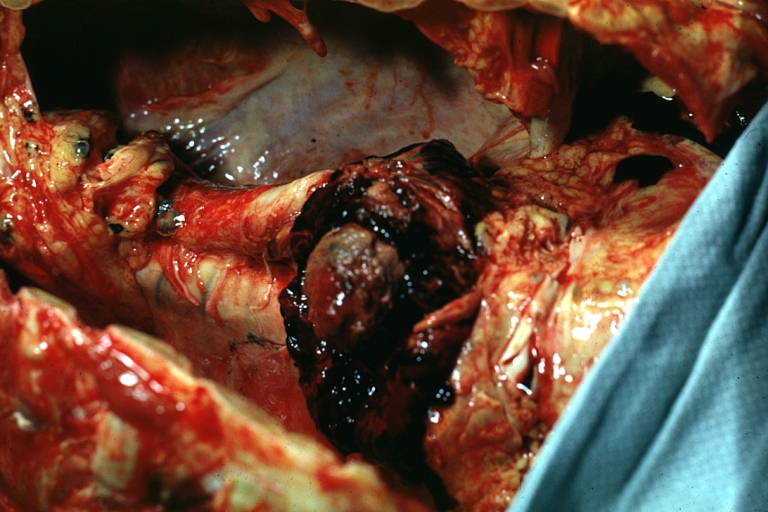
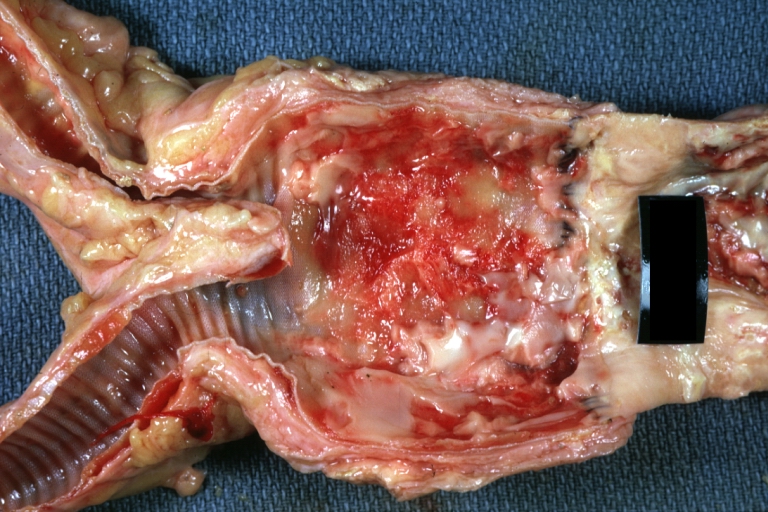
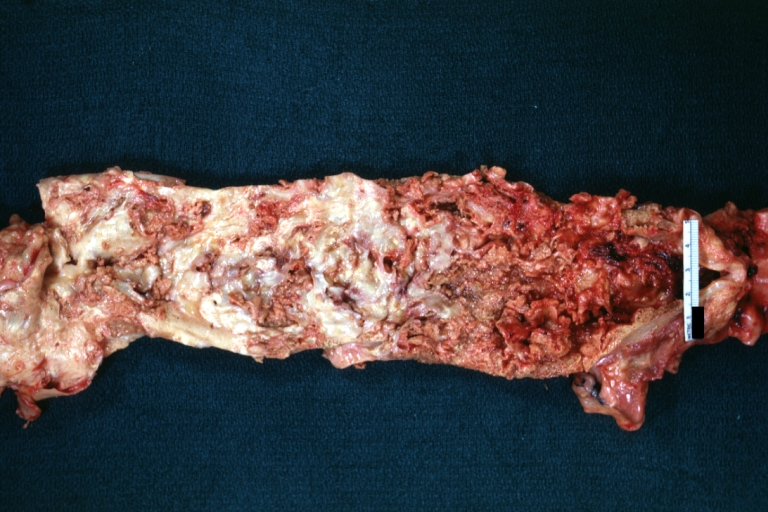
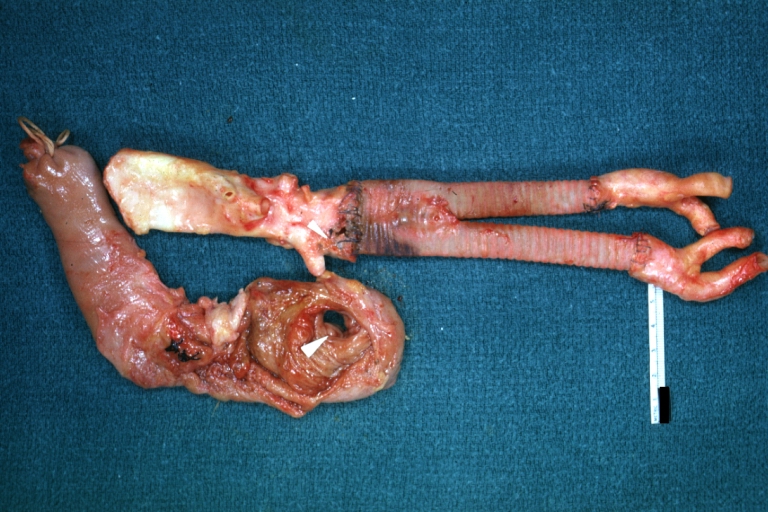
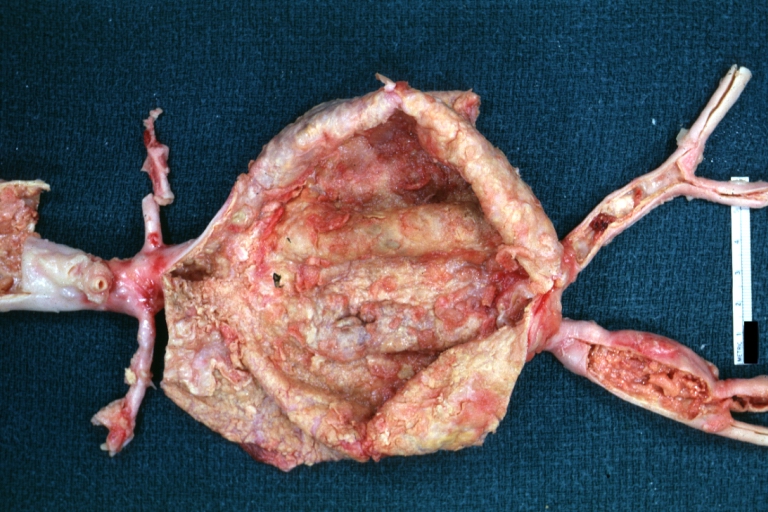
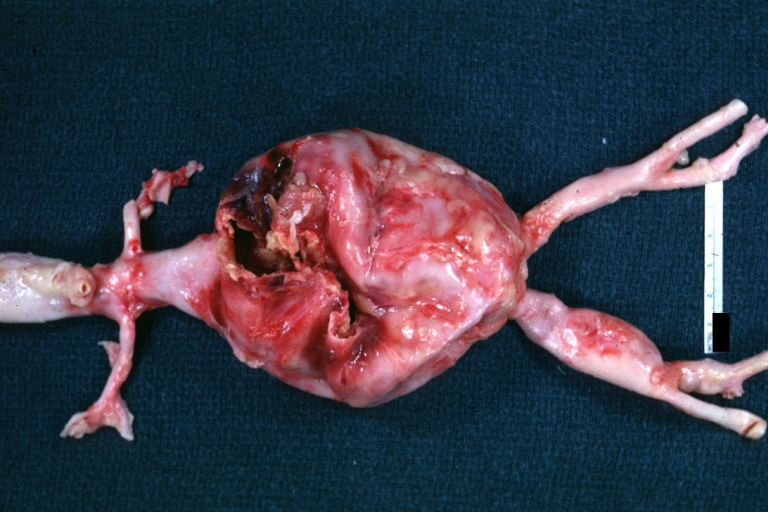
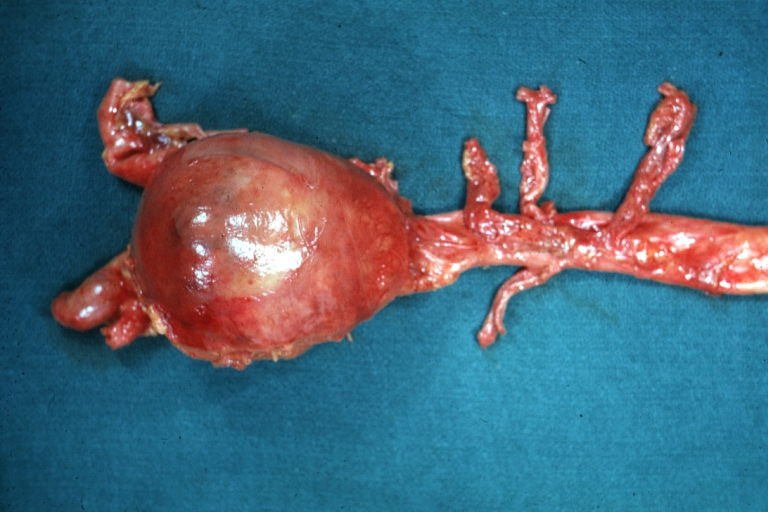
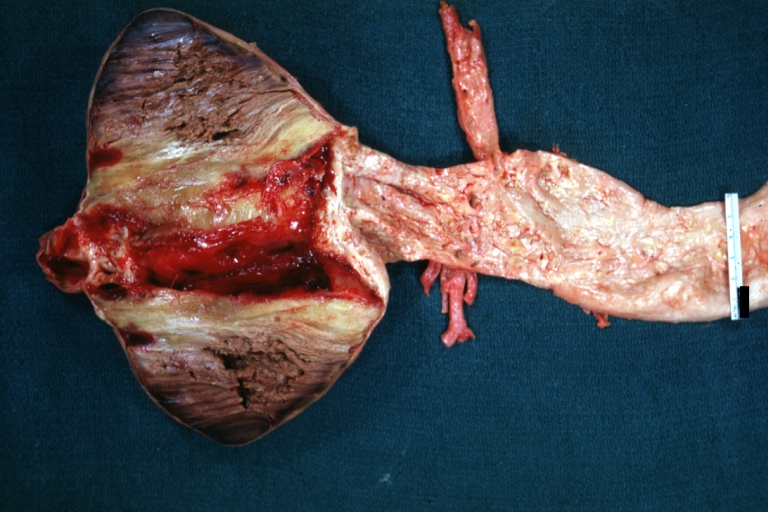
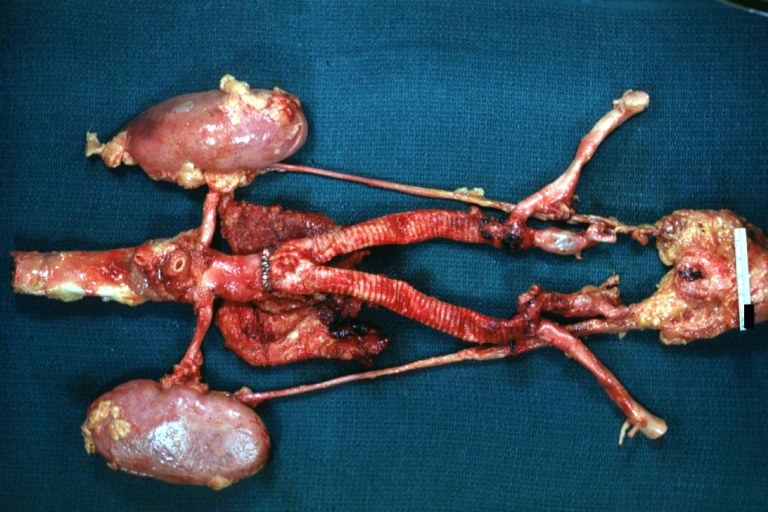
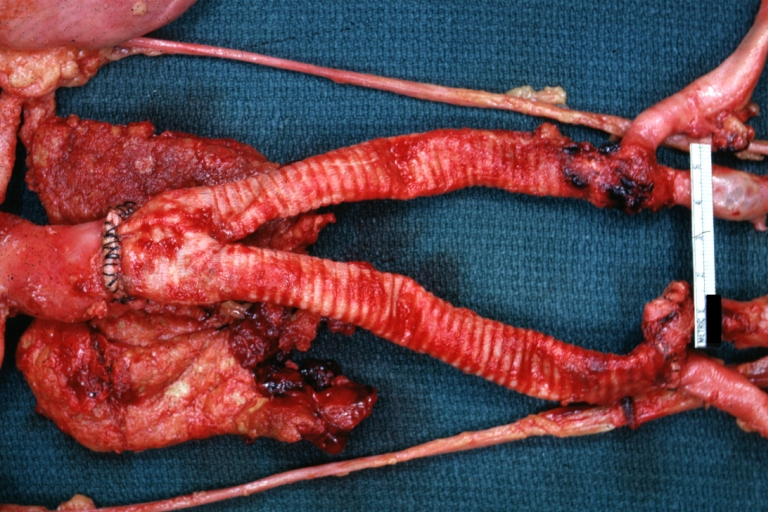
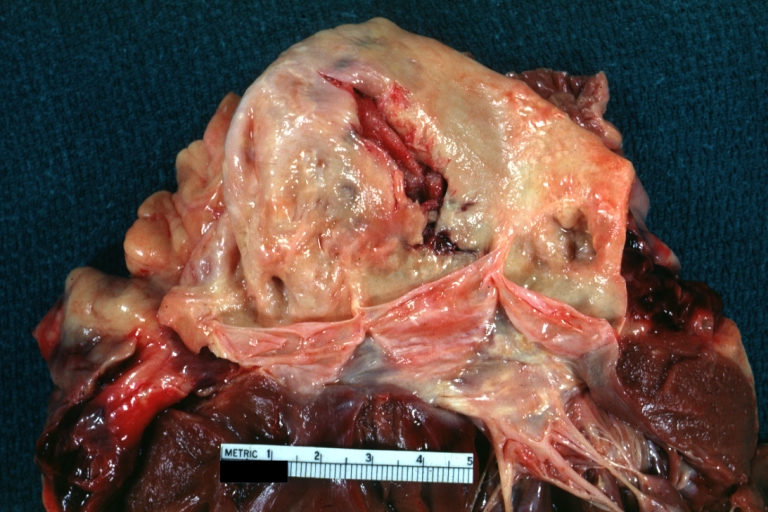
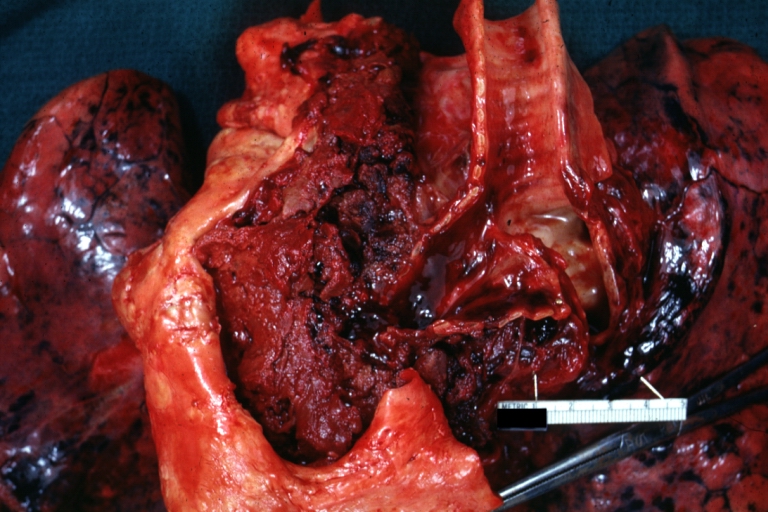
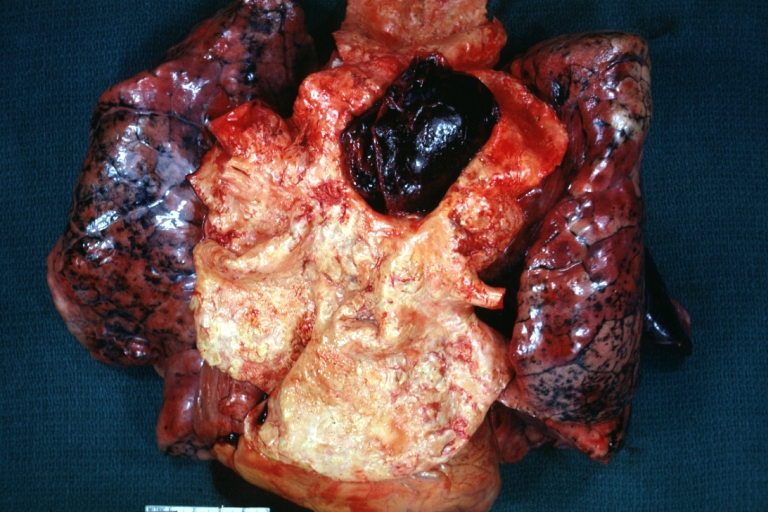
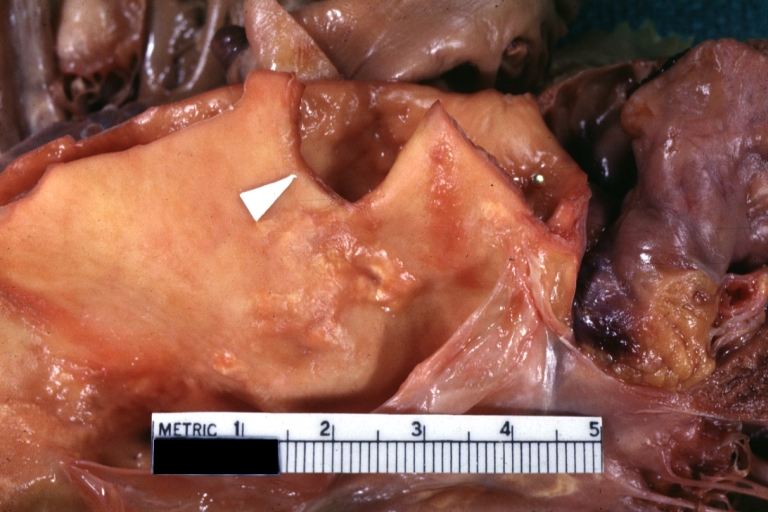
Gross Pathology
Microscopic Pathology
On microscopic histopathological analysis, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
-
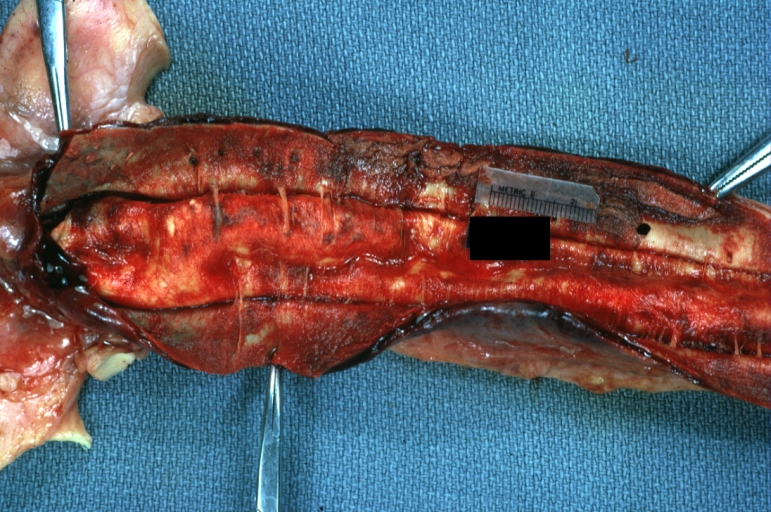
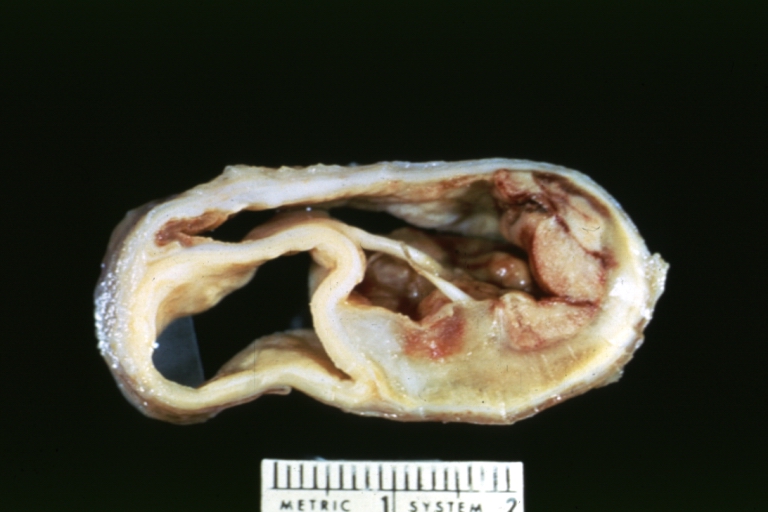
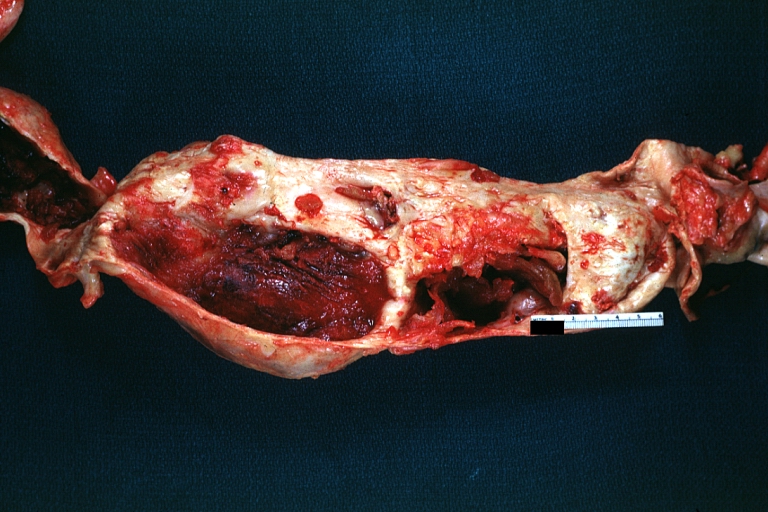
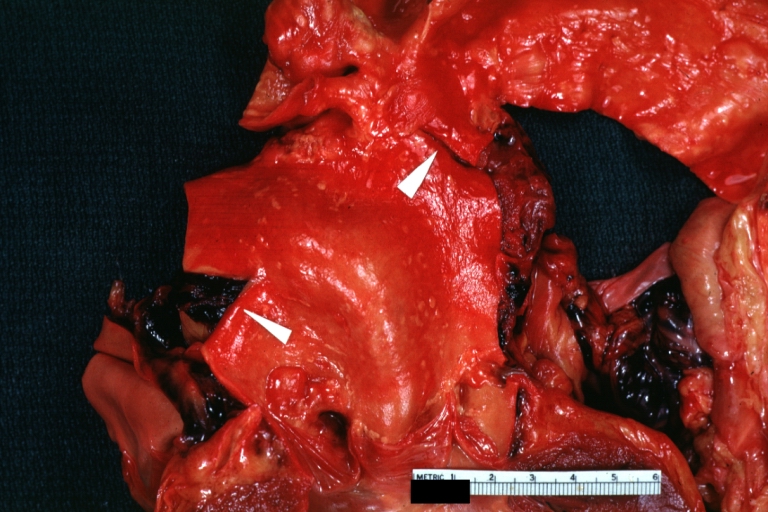
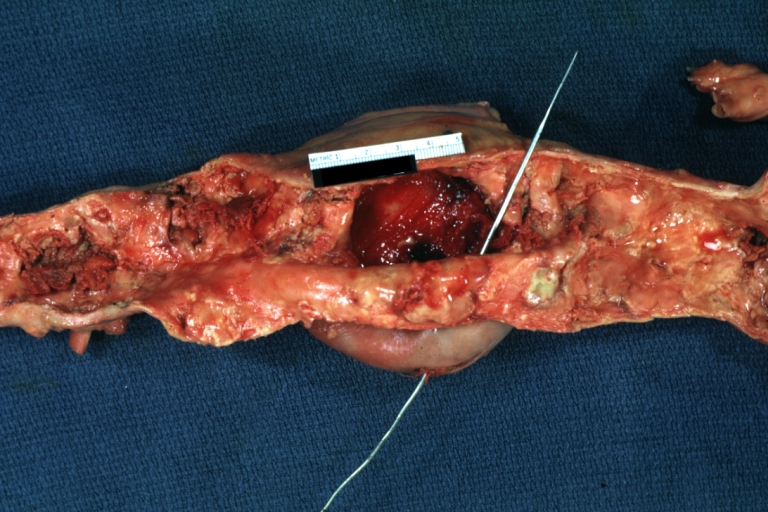
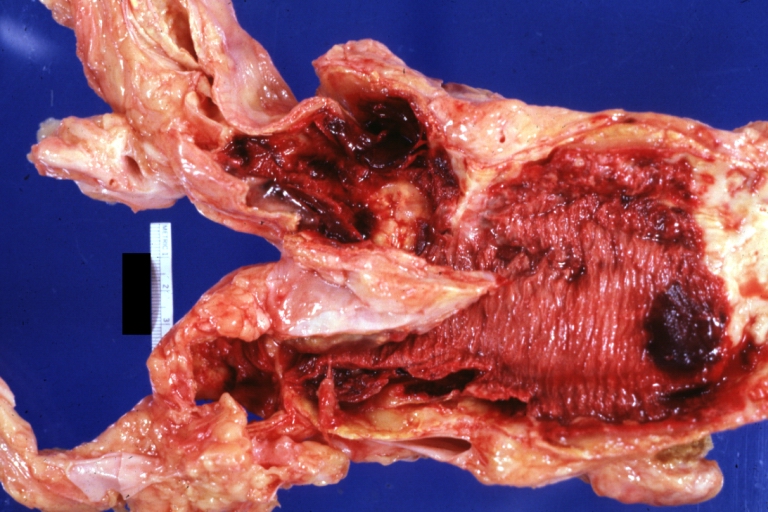
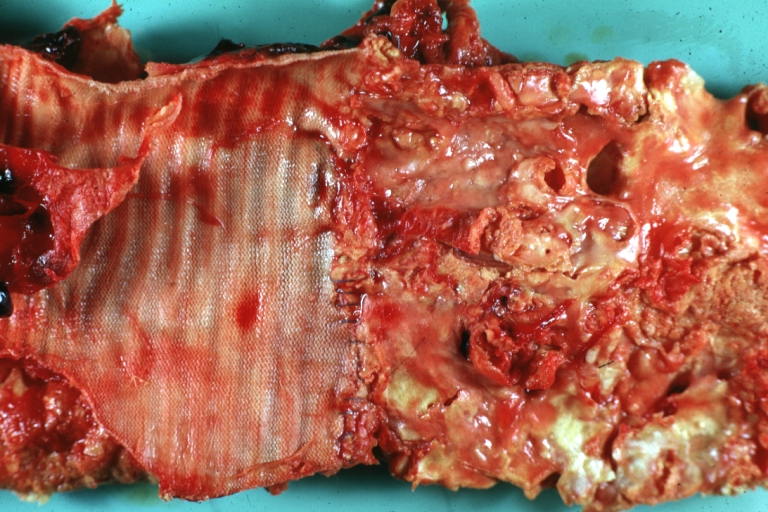
Dissecting Aneurysm: Gross very good example dissected channel has been opened
-
Dissecting Aneurysm: Gross external view good appearance from adventitia
-
Dissecting Aneurysm: Gross opened false channel
-
Dissecting Aneurysm: Gross good example dissection beginning at third portion aortic arch
-
Dissecting Aneurysm: Gross cross sections showing thrombus in false lumen true lumen has been opened longitudinally
-
Dissecting Aneurysm: Gross shows origin just above aortic valve false channel shown in descending thoracic aorta (very good example)
-
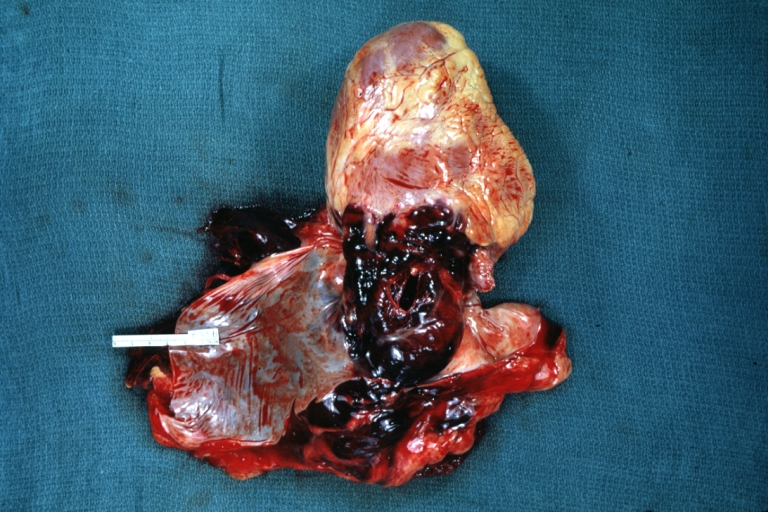
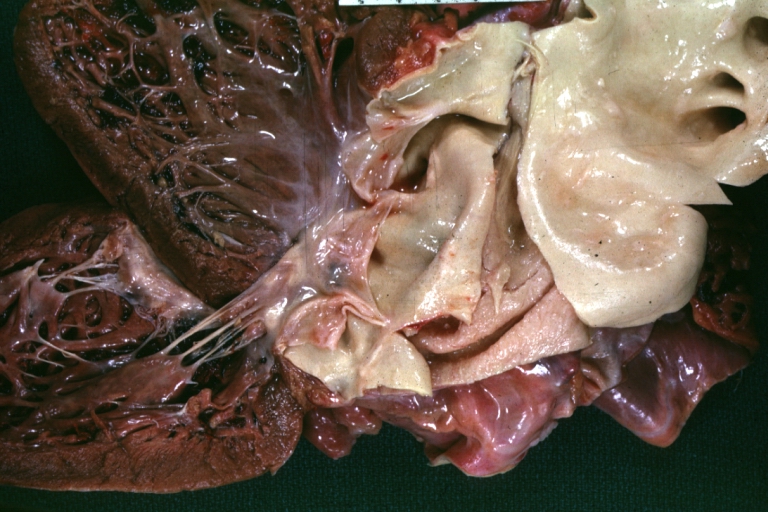
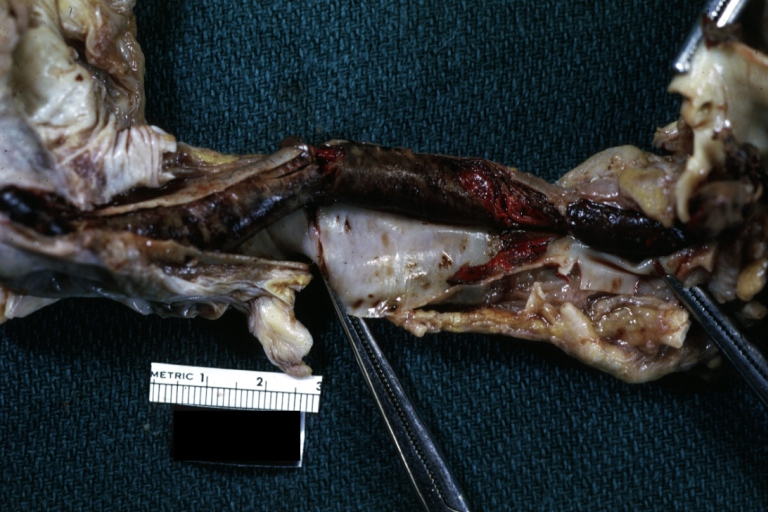
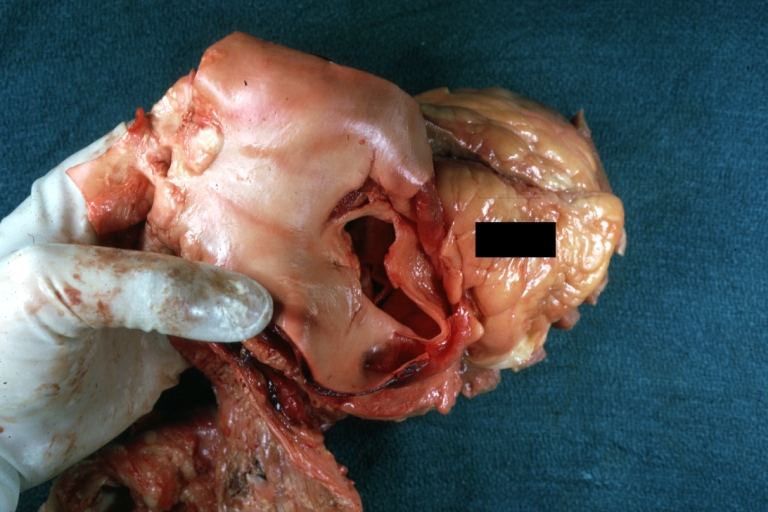
Atherosclerotic Aneurysm: Gross, a good example of typical abdominal aorta aneurysm with mural thrombus
-
Dissecting Aneurysm: Gross, a very good example of dissection beginning just above aortic ring
-
Atherosclerotic Aneurysm: Gross, (rather) good example of abdominal aortic aneurysm
-
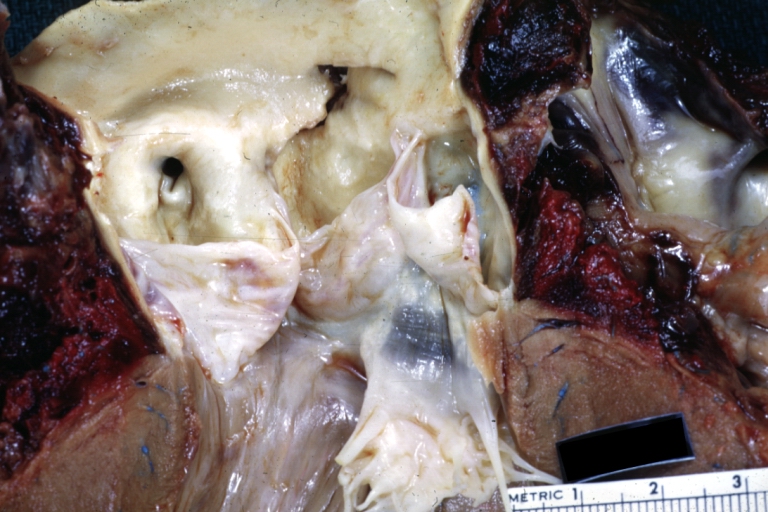
Dissecting Aneurysm: Gross, an excellent example, starting just above the aortic valve with reflection of aorta to show the dissection tract and some thrombus
-
Dissecting Aneurysm: Gross shows dilated aorta with extensive atherosclerosis dissection is seen, a small abdominal aorta atherosclerotic aneurysm is present good for association of dilation with dissection
-
Dissecting Aneurysm: Gross arrow points to start of dissection in first portion aortic arch good but not the best example shows dilation
-
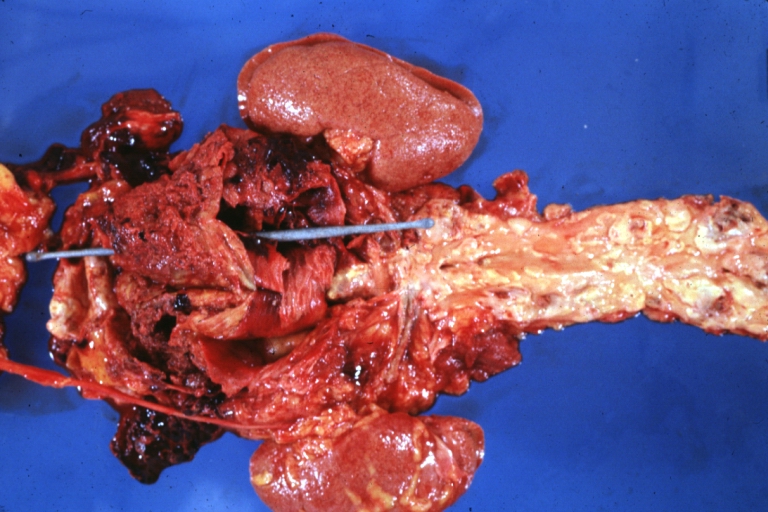
Dissecting Aneurysm: Gross, very good to show start of dissection above aortic valve and blood in false channel
-
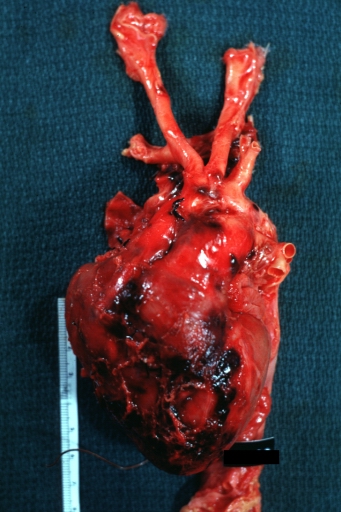
Dissecting Aneurysm: Gross, heart with root of aorta to show hemorrhage into pericardium (a very good example)
-
Dissecting Aneurysm: Gross, of heart and aorta with dissection and large false channel (a good example)
-
Dissecting Aneurysm: Gross cross section of aorta with two channels (a good example)
-
Atherosclerotic Aneurysm: Gross, a nice view of cross section of abdominal aorta aneurysm
-
Dissecting Aneurysm: Gross good example of typical angular tear above aortic valve
-
Dissecting Aneurysm: Gross good example angular tear above aortic valve
-
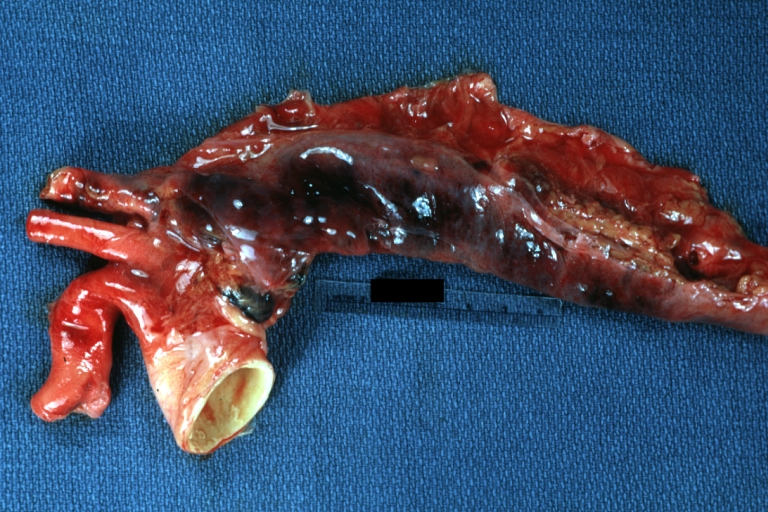
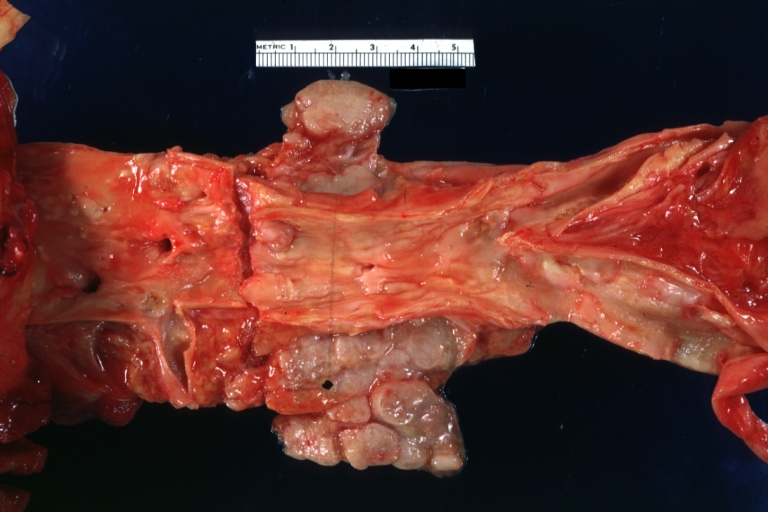
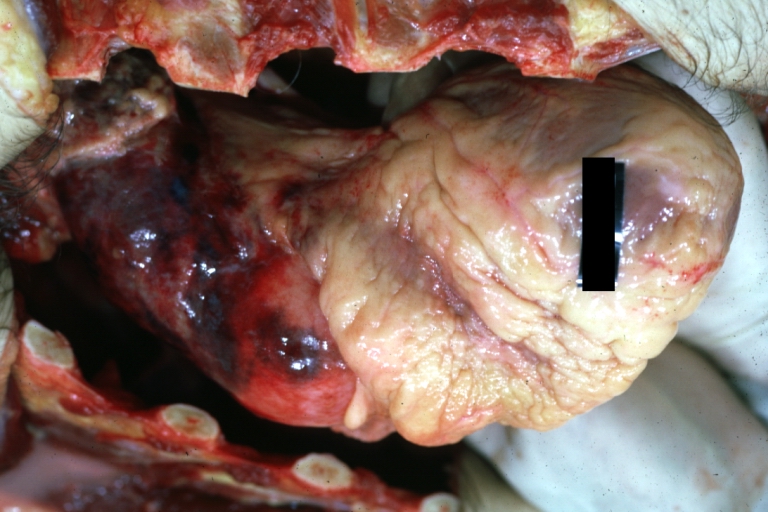
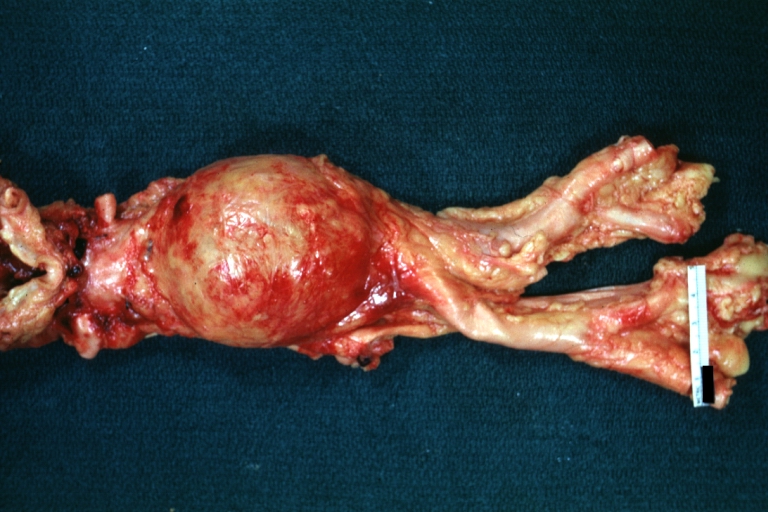
Atherosclerotic Aneurysm: Gross, external natural color very good example of an atherosclerotic thoracic aorta aneurysm with focal rupture
-
Atherosclerotic Aneurysm: Gross, excellent color, opened thoracic segment of aorta with two saccular atherosclerotic ruptured aneurysms
-
Atherosclerotic Aneurysm: Gross, an excellent example, natural color, external view of typical thoracic aortic aneurysms
-
Atherosclerotic Aneurysm: Gross unopened lesion natural color
-
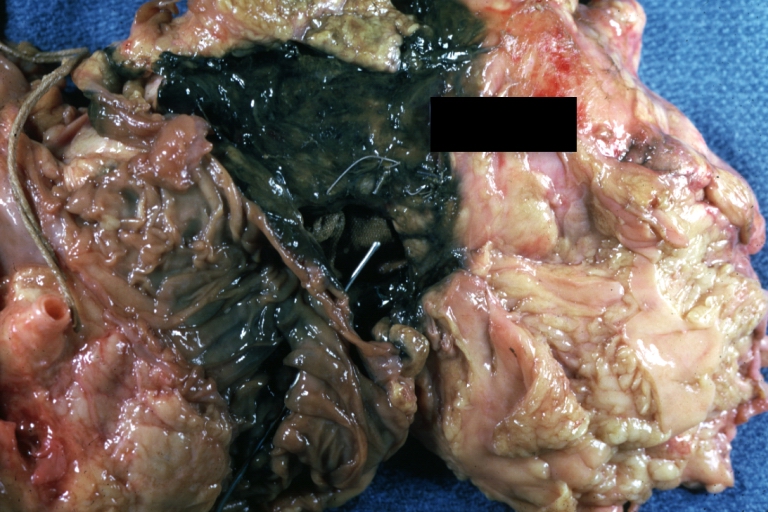
Dissecting Aneurysm: Gross dissection first portion of arch fixed specimen (a good example)
-
Dissecting Aneurysm: Gross, rather well shown dissection in first portion of the aortic arch
-
Dissecting Aneurysm: Gross, rather well shown dissection in first portion of the aortic arch
-
Dissecting Aneurysm: Gross, an excellent example of type I lesion
-
Dissecting Aneurysm: Gross, external view, an excellent example
-
Dissecting Aneurysm: Gross, Type I shows false channel
-
Dissecting Aneurysm: Gross, opened to show false channel (good example)
-
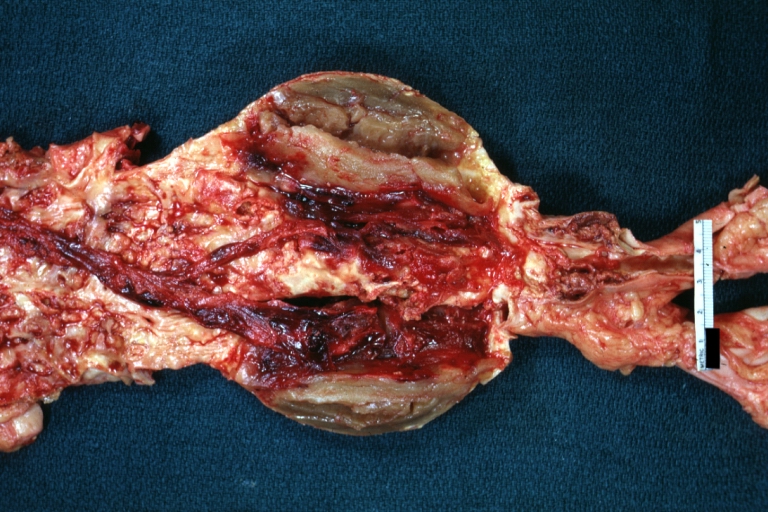
Atherosclerotic Aneurysm: Gross, very good example of ruptured thoracic segment
-
Dissecting Aneurysm: Gross, coagulum of blood in false channel
-
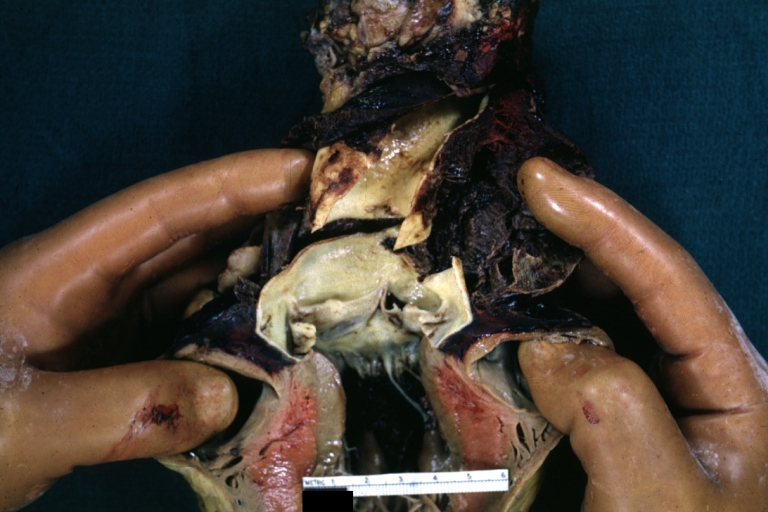
Dissecting Aneurysm: Gross, aortic valve area dissection (well shown, typical lesion)
-
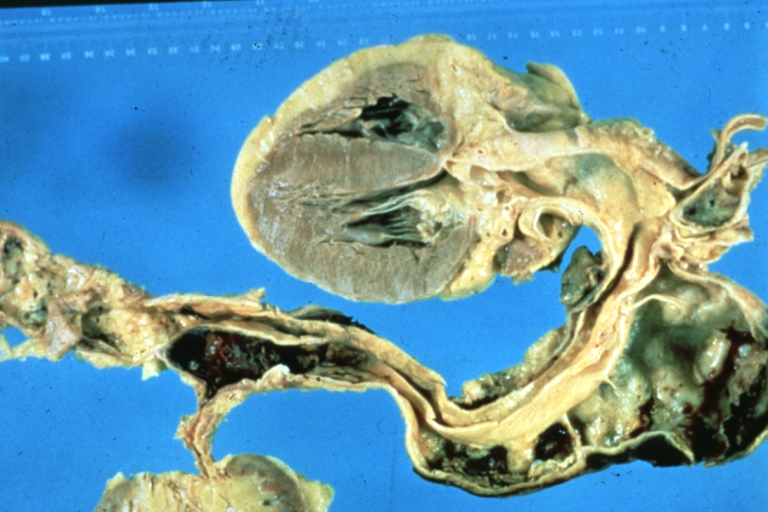
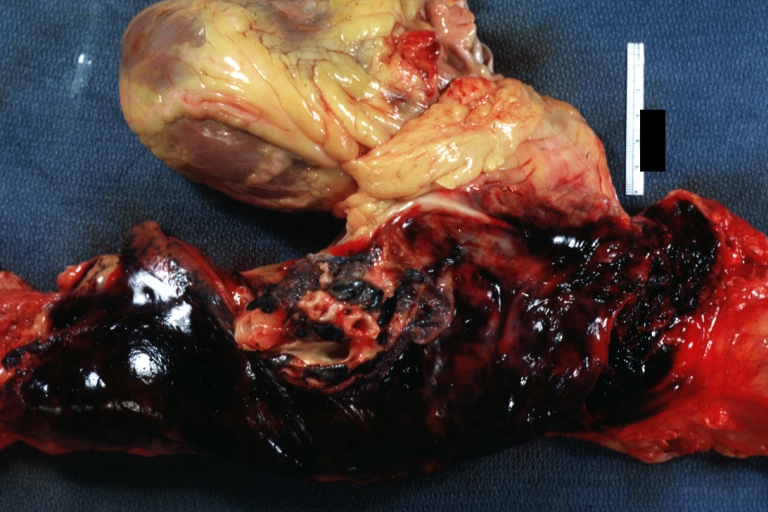
Abdominal Aneurysm Ruptured: Gross (good example) opened kidneys in marked place, atherosclerosis in lower thoracic aorta
-
Abdominal Aneurysm: Gross, (very good example) opened lesion with mural thrombus
-
Dissecting Aneurysm: Gross, large tear in first portion of aortic arch, annuloaortic ectasis
-
Dissecting Aneurysm: Gross, external view of heart and first portion of aortic arch, annuloaortic ectasia, hemorrhage beneath adventitia is evidence of dissection
-
Atherosclerotic Aneurysm Infected: Gross, infected abdominal aneurysm at superior suture line with rupture into duodenum
-
Atherosclerotic Aneurysm: Gross, cross sections of repaired aneurysm showing Dacron graft and old mural thrombus. A nice example of fibrin layer in graft
-
Ruptured Syphilitic Aneurysm
-
Dissecting Aneurysm in a patient with Marfan's syndrome
-
Traumatic Aneurysm
-
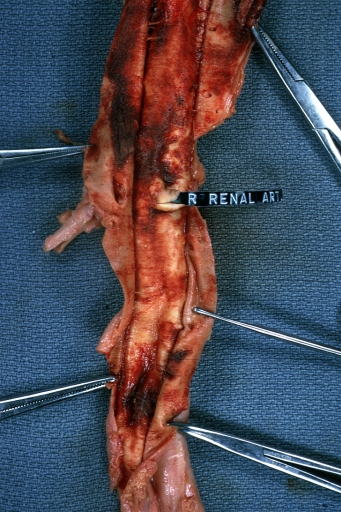
Kidney: Arteriosclerosis: Gross aorta with well shown renal artery containing large plaque and kidney with multiple cortical scars and atrophy also abdominal aorta aneurysm with mural thrombus (excellent example for renovascular hypertension)
-
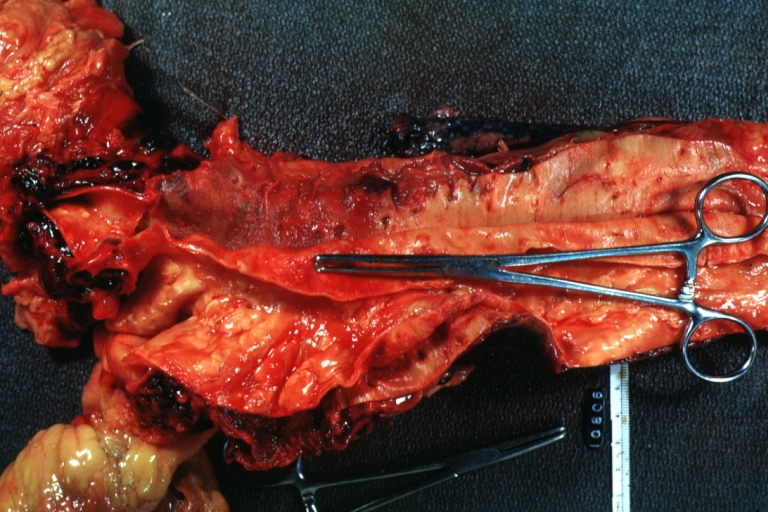
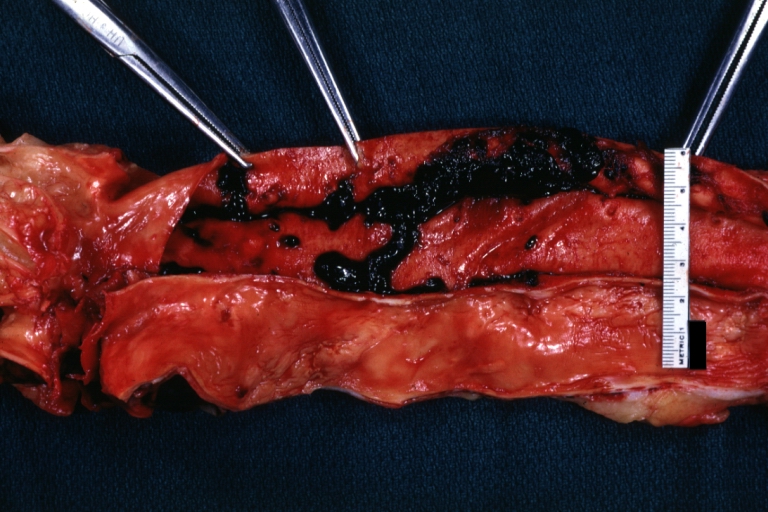
Dissecting Aneurysm: Gross, fixed tissue, descending thoracic segment dissection opened to show the false channel. The true surface is also visible
-
Aneurysm: Gross, ruptured thoracic aorta aneurysm, in situ lower thoracic portion (probably due to atherosclerosis)
-
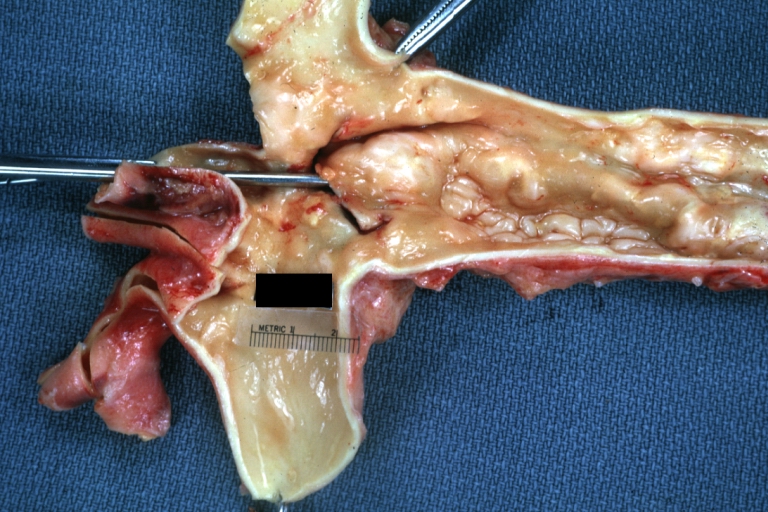
Abdominal Aneurysm Graft Repair: Gross, natural color, close-up view, an excellent example of Dacron graft that has been in place for years with pseudointima and atherosclerosis
-
Dacron Graft: Gross, close-up Dacron graft to repair aneurysm. Aorta completely covered with a calcified and ulcerated plaque with small mural thrombi (an excellent depiction of proximal suture line)
-
Dissecting Aneurysm: Gross natural color descending aorta opened into false channel
-
Abdominal Aneurysm: Gross, natural color, unopened specimen with about a six centimeter aneurysm between renals and bifurcation (a very good example of opened aneurysm)
-
Abdominal Aneurysm: Gross, natural color, an opened aneurysm showing quite well laminated thrombus
-
Atherosclerosis with Mural Thrombi: Gross, natural color, a nice photo of descending thoracic aorta with extensive ulcerated plaques and mural thrombi in distal portion. The case also has an abdominal aneurysm
-
Pseudoaneurysm Ruptured Into Duodenum: Gross natural color aorta and duodenum with arrow pointing to rupture point of aortobifemoral bypass pseudoaneurysm rupture and another in duodenum a very good demonstration of this very well known complication of aortic prostheses
-
Abdominal Aneurysm: Gross, natural color, large aneurysm opened showing sessile calcified plaques with no mural thrombus. Lesion extends from renal arteries to the bifurcation (the same lesion seen externally with focus of rupture)
-
Abdominal Aneurysm Ruptured: Gross, natural color, external view with large area of apparent rupture. Aorta is opened to show this aneurysm)
-
Abdominal Aneurysm: Gross, natural color, unopened large and quite typical aneurysm extending from below renal arteries to bifurcation
-
Abdominal Aneurysm: Gross, natural color, opened aneurysm with well shown and typical laminated thrombus (external view)
-
Aortobifemoral Prosthesis: Gross, natural color, nice dissection showing Dacron prosthesis replacing abdominal segment of aorta with portion of atherosclerotic aneurysm with renal arteries and kidneys
-
Aortobifemoral Prosthesis: Gross natural color close-up view of nicely dissected prosthesis extending from below renals to common iliac arteries portion of atherosclerotic aneurysm behind prosthesis
-
Dissecting Aneurysm: Gross natural color close-up view of aortic valve and proximal aortic arch with ruptured intima rather good illustration of this lesion
-
Syphilitic Aneurysm: Gross natural color rather a close-up view and outstanding photo of aneurysm ruptured into the left main stem bronchus
-
Syphilitic Aneurysm: Gross natural color typical tree barking in aorta aneurysm opening is seen in which is a thrombus aneurysm ruptured into left main stem bronchus (shown very well)
-
Dissecting Aneurysm Chronic: Gross natural color first portion of aortic arch with intimal rent well shown with healed margins and view into false channel that shows a surface looking like atherosclerosis which is known to develop in a chronic dissection
-
Dissecting Aneurysm Chronic: Gross, natural color, closer view of the previous one (a very good example)
References
- ↑ Coady MA, Rizzo JA, Elefteriades JA (November 1999). "Developing surgical intervention criteria for thoracic aortic aneurysms". Cardiol Clin. 17 (4): 827–39. doi:10.1016/s0733-8651(05)70118-1. PMID 10589349.
- ↑ Ruddy JM, Jones JA, Spinale FG, Ikonomidis JS (November 2008). "Regional heterogeneity within the aorta: relevance to aneurysm disease". J. Thorac. Cardiovasc. Surg. 136 (5): 1123–30. doi:10.1016/j.jtcvs.2008.06.027. PMC 2679174. PMID 19026791.
- ↑ Castellano JM, Kovacic JC, Sanz J, Fuster V (April 2012). "Are we ignoring the dilated thoracic aorta?". Ann. N. Y. Acad. Sci. 1254: 164–74. doi:10.1111/j.1749-6632.2012.06493.x. PMID 22548582.
- ↑ Wolinsky H (October 1970). "Comparison of medial growth of human thoracic and abdominal aortas". Circ. Res. 27 (4): 531–8. doi:10.1161/01.res.27.4.531. PMID 5507030.
- ↑ Halloran BG, Davis VA, McManus BM, Lynch TG, Baxter BT (July 1995). "Localization of aortic disease is associated with intrinsic differences in aortic structure". J. Surg. Res. 59 (1): 17–22. doi:10.1006/jsre.1995.1126. PMID 7630123.
- ↑ Steed MM, Tyagi SC (October 2011). "Mechanisms of cardiovascular remodeling in hyperhomocysteinemia". Antioxid. Redox Signal. 15 (7): 1927–43. doi:10.1089/ars.2010.3721. PMC 3159179. PMID 21126196.
- ↑ Zhou J, Austin RC (2009). "Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms". Biofactors. 35 (2): 120–9. doi:10.1002/biof.17. PMID 19449439.
- ↑ Steed MM, Tyagi N, Sen U, Schuschke DA, Joshua IG, Tyagi SC (September 2010). "Functional consequences of the collagen/elastin switch in vascular remodeling in hyperhomocysteinemic wild-type, eNOS-/-, and iNOS-/- mice". Am. J. Physiol. Lung Cell Mol. Physiol. 299 (3): L301–11. doi:10.1152/ajplung.00065.2010. PMC 2951073. PMID 20581102.
- ↑ Dalton ML, Gadson PF, Wrenn RW, Rosenquist TH (July 1997). "Homocysteine signal cascade: production of phospholipids, activation of protein kinase C, and the induction of c-fos and c-myb in smooth muscle cells". FASEB J. 11 (8): 703–11. doi:10.1096/fasebj.11.8.9240971. PMID 9240971.
- ↑ Jones JA, Barbour JR, Lowry AS, Bouges S, Beck C, McClister DM, Mukherjee R, Ikonomidis JS (December 2006). "Spatiotemporal expression and localization of matrix metalloproteinas-9 in a murine model of thoracic aortic aneurysm". J. Vasc. Surg. 44 (6): 1314–21. doi:10.1016/j.jvs.2006.07.042. PMC 1761919. PMID 17145436.
- ↑ Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A (September 2011). "Prolonged infusion of angiotensin II in apoE(-/-) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm". Am. J. Pathol. 179 (3): 1542–8. doi:10.1016/j.ajpath.2011.05.049. PMC 3157213. PMID 21763672.