Tetracaine (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tetracaine (ophthalmic) is a local anesthesic that is FDA approved for the procedure of tonometry, gonioscopy, removal of corneal foreign bodies, conjunctival scraping for diagnostic purposes, suture removal from the cornea or conjunctiva, other short corneal and conjunctival procedures. Common adverse reactions include nausea, vomiting, and burning sensation in eye.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
For Tonometry and Other Procedures of Short Duration
- Instill one or two drops just prior to evaluation.
For Minor Surgical Procedures such as Foreign Body or Suture Removal
- Instill one or two drops every five to ten minutes for one to three doses.
For Prolonged Anesthesia as in Cataract Extraction
- Instill one or two drops in the eye(s) every five to ten minutes for three to five doses.
Epidural Anesthesia
- As with all anesthetics, the dosage varies and depends upon the area to be anesthetized, the number of neuronal segments to be blocked, individual tolerance, and the technique of anesthesia. The lowest dosage needed to provide effective anesthesia should be administered. For specific techniques and procedures, refer to standard textbooks.

- The extent and degree of spinal anesthesia depend upon dosage, specific gravity of the anesthetic solution, volume of solution used, force of the injection, level of puncture, position of the patient during and immediately after injection, etc.
- When spinal fluid is added to 1% tetracaine hydrochloride injection, some turbidity results, the degree depending on the pH of the spinal fluid, the temperature of the solution during mixing, as well as the amount of drug and diluent employed. Liberation of base (which is completed within the spinal canal) is held to be essential for satisfactory results with any spinal anesthetic.
- The specific gravity of spinal fluid at 25°C/25°C varies under normal conditions from 1.0063 to 1.0075. The 1% concentration in saline solution has a specific gravity of 1.0060 to 1.0074 at 25°C/25°C.
- A hyperbaric solution may be prepared by mixing equal volumes of the 1% solution and Dextrose Solution 10%.
- Examine ampules carefully before use. Do not use solution if crystals, cloudiness, or discoloration is observed.
This formulation of tetracaine hydrochloride does not contain antimicrobial or bacteriostatic agents; therefore, unused portions should be discarded.
Sterilization of Ampules
- The tetracaine hydrochloride injection is sterile within an undamaged ampule. To destroy bacteria on the exterior of ampules use heat sterilization (autoclaving) before opening. Immersion in antiseptic solution is not recommended.
Autoclave at 15-pounds pressure, at 121°C (250°F), for 15 minutes. Autoclaving increases likelihood of crystal formation. Unused autoclaved ampules should be discarded. Under no circumstances should unused ampules which have been autoclaved be returned to stock.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tetracaine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tetracaine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Tetracaine (ophthalmic) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tetracaine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tetracaine in pediatric patients.
Contraindications
- Should not be used by the patient without physician supervision, or in those persons showing hypersensitivity to any component of this preparation.
Warnings
- For topical ophthalmic use only. Not for parenteral use. Not for injection. Do not use solution if it contains crystals or if it is cloudy or discolored. Prolonged use results in diminished duration of anesthesia and retarded healing. This may cause the drug to be used more frequently, creating a “vicious circle”. Subsequent corneal infection and/or corneal opacification with accompanying permanent visual loss or corneal perforation may occur. Prolonged use may also produce severe keratitis.
Adverse Reactions
Clinical Trials Experience
- Transient symptoms (signs) such as stinging, burning and conjunctival redness may occur. A rare, severe, immediate type allergic corneal reaction has been reported characterized by acute diffuse epithelial keratitis with filament formation and/or sloughing of large areas of necrotic epithelium, diffuse stromal edema, descemetitis and iritis.
TO REPORT SUSPECTED ADVERSE REACTIONS, contact Altaire Pharmaceuticals, Inc., at 1-800-258-2471 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Postmarketing Experience
There is limited information regarding Tetracaine (ophthalmic) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Tetracaine (ophthalmic) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been performed with Tetracaine Hydrochloride. It is also not known whether Tetracaine Hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% should be given to pregnant women only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tetracaine (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tetracaine (ophthalmic) during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is administered to a nursing mother.
Pediatric Use
- Safety and effectiveness in children have not been established.
Geriatic Use
There is no FDA guidance on the use of Tetracaine (ophthalmic) in geriatric settings.
Gender
There is no FDA guidance on the use of Tetracaine (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tetracaine (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tetracaine (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tetracaine (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tetracaine (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tetracaine (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Tetracaine (ophthalmic) Administration in the drug label.
Monitoring
There is limited information regarding Tetracaine (ophthalmic) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tetracaine (ophthalmic) and IV administrations.
Overdosage
There is limited information regarding Tetracaine (ophthalmic) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Tetracaine (ophthalmic)
| |
| Systematic (IUPAC) name | |
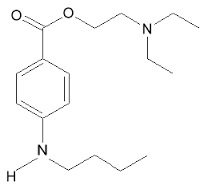
| 2-(dimethylamino)ethyl 4-(butylamino)benzoate | |
| Identifiers | |
| CAS number | 136-47-0 (hydrochloride) |
| ATC code | C05 D04AB06 (WHO) N01BA03 (WHO) S01HA03 (WHO) |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 264.363 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 75.6 |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Rx Only |
| Routes | Topical, Epidural, Spinal |
Mechanism of Action
There is limited information regarding Tetracaine (ophthalmic) Mechanism of Action in the drug label.
Structure
- Chemical Name: Benzoic acid, 4-(butylamino)-,2-(dimethylamino)ethyl ester, monohydrochloride

Pharmacodynamics
- Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% acts by decreasing the permeability of the neuronal membrane, thereby decreasing the flux of sodium, potassium and other ions associated with propagation of the nerve impulse. The onset of anesthesia usually begins within 30 seconds and lasts a relatively short period of time.
Pharmacokinetics
There is limited information regarding Tetracaine (ophthalmic) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Tetracaine (ophthalmic) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Tetracaine (ophthalmic) Clinical Studies in the drug label.
How Supplied
- Tetracaine Hydrochloride Ophthalmic Solution, USP 0.5% is supplied in 15 mL and 30 mL single drop plastic containers.
Storage
- Store at room temperature, 15°-30°C (59°-86°F). Keep container tightly closed. Protect from light.
Images
Drug Images
{{#ask: Page Name::Tetracaine (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tetracaine (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Tetracaine (ophthalmic) Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Tetracaine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Tetracaine (ophthalmic) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Tetracaine (ophthalmic) |Label Name=Tetracaine Package.png
}}